Download PDF
This past November, the Academy celebrated the 20th anniversary of the Spotlight on Cataract Surgery with a return to a live event at AAO 2021. We brought back the popular case-based format, “Clinical Decision-Making With Cataract Complications: You Make the Call.” Cochaired by Nicole R. Fram, MD, and myself, this four-hour symposium was organized around eight cases that presented a range of cataract surgical challenges and complications.
The cases were selected from our own practices and, as we presented the videos, we paused at selected points that highlighted a complication or the need to make a management decision. The attendees were then asked to register their clinical decisions using their mobile devices. This was followed by several rapid-fire didactic presentations by invited experts on topics of relevance to the case. Next, a rotating panel of two discussants (who had never viewed the case) was asked to make a management recommendation before the video of the outcome was shown. Following additional audience polling about preferences and practices, the two panelists provided their own opinions and pearls.
In all, nearly 40 presenters and panelists spoke about a wide variety of topics, including white, rock-hard, and traumatic cataracts, small pupils, crowded anterior segments, phacodonesis, posterior capsular rupture with posterior polar and ultrabrunescent cataracts, vitreous prolapse through a zonular dialysis, unhappy diffractive IOL patients, complex IOL exchange, IOL opacification and subluxation, and bag/IOL dislocation of a high-power toric IOL. Michael E. Snyder, MD, concluded the session by delivering the 16th Annual Charles Kelman Lecture, “Niche Devices for Special Eyes.”
This EyeNet article reports the results of the 33 audience response questions, accompanied by written commentary from the speakers and panelists. The polled respondents included both the live and virtual audiences. Because of the anonymous nature of this polling method, the audience opinions are always candid and were discussed in real time during the event by our panelists.
View the videos at aao.org/cataract-spotlight-aao2021 or see below for instructions on viewing the full symposium. My reflections on the 20-year history of the Cataract Spotlights can be found at aao.org/eyenet/academy-live/detail/20-years-of-cataract-spotlights.
—David F. Chang, MD
Cataract Spotlight Program Cochairman
Case 1: White Lens + Shallow AC + Fixed Pupil
This 83-year-old patient had a prior peripheral iridotomy for narrow angles. Her IOP is 4 mm Hg, thanks to a prior trabeculectomy. After delaying any eye exams due to COVID, she presented with a white lens, an extremely shallow anterior chamber (AC), and a fixed small pupil due to posterior synechiae.
Q1.1 What is your preferred capsulotomy method for a white lens?
| Irrigating cystotome after dye staining |
34.4%
|
| Forceps after dye staining |
18.8%
|
|
Forceps after dye staining and cortical aspiration
|
40.6%
|
|
Femto capsulotomy
|
6.3%
|
|
Zepto or other technology/method
|
0%
|
Eric Donnenfeld The overwhelming key to successful removal of a white cataract is the capsulotomy, which can be the most challenging aspect of the case. The capsule is difficult if not impossible to visualize without dye staining, which was recommended by essentially all respondents. In addition, high intracapsular pressure due to liquified lens protein can result in the classic radial capsular tear (i.e., the “Argentinian flag” sign).
Understanding the pathophysiology of the white cataract is crucial to effective management. The surgeon should be prepared to take three key steps: 1) decreasing vitreous and intracapsular pressure while 2) maintaining or increasing AC pressure, in order to 3) prevent the capsule from bowing forward and tearing. The use of trypan blue to help with visualization has been a major improvement in managing these cases, and the surgeon should maximally fill the AC with a high–molecular weight viscoelastic agent to flatten the capsule, thereby reducing capsular tension and maintaining the pressure gradient between the AC and the lens. Preoperative mannitol can also be a helpful adjunct.
The incision into the AC should be as small as possible and be beveled and self-sealing to avoid wound leakage. As the surgeon performs the capsulorrhexis, care should be taken to prevent burping of viscoelastic or aqueous through the corneal incision, as this would alter the AC equilibrium. It is always better to begin with a smaller capsulorrhexis to prevent radial tears; the capsulorrhexis can be enlarged later prior to removal of the lens and can even be done after the IOL has been placed.
When intralenticular pressure appears very high, aspiration of the liquid cortex with a 25- or 27-gauge needle following staining of the capsule and insertion of viscoelastic may help decompress the capsular bag. This was recommended by 40% of respondents. On the other hand, I would recommend caution with an irrigating cystotome (recommended by 34% of respondents), as it may evacuate the viscoelastic, thus causing a sudden lowering of AC pressure. I generally prefer a traditional nonirrigating cystotome.
The use of femtosecond laser–assisted cataract surgery (FLACS) has several advantages in these cases, in that the AC is closed and IOP is maintained. The key to a femto capsulorrhexis is that the capsulotomy should take place as quickly as possible; this keeps the milky material from blocking the laser pulses. In any event, the capsule should be stained with trypan blue, and it should be removed with the assumption that it has residual tags, which are present in approximately 20% of patients.
The femto capsulotomy has been shown to reduce the incidence of the Argentinian flag sign. However, in this particular case, FLACS would be less advantageous due to the small, fixed pupil. Instantaneous creation of the entire capsulorrhexis with another new technology, Zepto (Centricity Vision), would avoid placing stress at the center of the lens, where intralenticular pressure is the highest.
In summary, the surgeon should come prepared with a plan. While this is a challenging procedure at times, restoring vision in a patient with a white cataract is one of the most gratifying procedures in our profession.
Q1.2 How would you manage the small pupil in this case?
| Would not mechanically enlarge |
1.9%
|
| Malyugin Ring |
51.9%
|
|
Other pupil expansion ring
|
3.7%
|
|
Iris retractors
|
33.3%
|
|
Stretch the pupil after lysing the posterior synechiae
|
9.3%
|
Deepinder K. Dhaliwal I agree with the audience that the pupil should be expanded for optimal visualization and safe surgery. What you don’t see during phacoemulsification can definitely hurt you!
The decision of whether to use a Malyugin Ring or iris retractors depends on the depth of the AC and concomitant ocular pathology. If the AC is very shallow, the case may be complex—and if the eye is very small, iris retractors are preferred, as the surgeon can adjust the location and number of the retractors based on the pathology. Otherwise, I agree with over half of the respondents in that I, too, prefer the Malyugin Ring. I find the ring to be efficient and effective. In addition, as it comes in both the 6.25- and 7-mm sizes, it is helpful even in cases of intraoperative floppy iris syndrome (IFIS), in which the pupil dilates moderately. Also, because the ring is inserted and removed through the main wound, it is time efficient as well.
Viscoelastic can be placed under the iris to aid in insertion of the ring. To make removal of the ring atraumatic, I disengage the distal scroll first, then the lateral scrolls. The subincisional scroll is disengaged last, then the entire ring is pushed away from the main wound. I use the insertion device to re-engage the ring, retracting it just until both lateral scrolls overlap. I then stop retracting the ring—instead, I pull the entire inserter out of the eye. This technique is atraumatic to the surrounding tissue (especially the corneal endothelium). Retracting the entire ring back into the inserter is unnecessary and may create challenges if it does not retract symmetrically.
Finally, mechanically lysing the posterior synechiae is an important step that can be achieved with a cyclodialysis spatula or the viscoelastic cannula. However, simply stretching the pupil in this case may not be sufficient.
Q1.3 How would you proceed with the white lens and small pupil in this case?
| Instill capsular dye before an ophthalmic viscoelastic device (OVD)—e.g., beneath an air bubble |
66.7%
|
| Instill capsular dye after OVD but before pupil expansion |
0%
|
|
Instill capsular dye after OVD and after pupil expansion
|
31%
|
|
Femto capsulotomy
|
2.4%
|
|
Other
|
0%
|
Thomas W. Samuelson Cataract surgery following trabeculectomy presents several unique considerations. Such patients often have poorly dilating pupils and denser cataracts. While more miotic pupils are common among individuals with glaucoma for a variety of reasons, including pseudoexfoliation (PEX), bound down pupils with posterior synechiae are very common following phakic trabeculectomy due to the associated iridectomy.
Moreover, cataract surgery is often delayed in patients with preexisting blebs to allow the bleb to mature and become well-established. Accordingly, such patients often have denser nuclear sclerotic lenses and more mature cortex. This is in distinct contrast to a current trend in glaucoma management, in which cataract surgery is often performed earlier to improve glaucoma control, generally combined with a minimally invasive glaucoma surgery (MIGS) procedure. Indeed, cataract surgery in patients with preexisting blebs is one of the few scenarios in which cataract surgery can be expected to raise IOP postoperatively due to subsequent bleb fibrosis and contraction. The subject of this case presentation also has relative hypotony, which often hastens cortical maturation.
My management in such cases falls in line with the audience response. That is, I utilize capsular dye and pupil expansion as needed to facilitate visualization. This is especially important when managing mature cortical cataracts, which have a higher propensity for “runout” during the capsulorrhexis. I will generally instill dye prior to the OVD. While the AC is filled with the dye, I will re-pressurize it with balanced salt solution (BSS). I then use the supraphysiologic hydrostatic pressure to push down on the iris, further expanding the pupil and dye coverage on the AC. Then, following irrigation of the dye from the AC, while the OVD is in place, I will use a Kuglen hook to break the posterior synechiae, further expanding the pupil. Additional capsular dye painted directly onto the lens capsule beneath the OVD will allow better staining in the periphery of the capsule.
If the pupil remains too miotic following these measures, I will use either an expansion ring or capsular hooks to improve visualization. I generally prefer the ring but will preferentially use hooks in very small eyes or when there is known zonular insufficiency. Once phacoemulsification begins, it is important to monitor the bleb and manage the irrigating fluid infusion pressure. I have had the experience of bleb rupture during phacoemulsfication—and if this occurs, one must be prepared for the small chance that a bleb revision may be needed.
Finally, surgeons must also be aware that the low preoperative IOP may result in a shorter axial length, thus influencing IOL calculations. If the postoperative IOP is higher than preoperative IOP, the axial length may increase, resulting in a myopic outcome. It is important to inform these patients that their surgery is more nuanced and to manage expectations. With proper pre- and intraoperative planning, such cases are very rewarding and may significantly improve quality of life.
Q1.4 How would you manage the very shallow AC in this eye?
| Manage using OVD only |
50.9%
|
| OVD plus digital massage |
1.8%
|
|
OVD plus IV manni
|
24.6%
|
|
OVD plus pars plana vitreous tap
|
3.5%
|
|
Options 3 and 4
|
19.3%
|
Doug Koch This case is challenging because of the combination of shallow AC and low IOP. Possible causes of the shallow chamber are aqueous misdirection, choroidal effusion, or massive lenticular swelling, but it is highly unlikely that the lens alone would create an “extremely shallow” chamber. To investigate further, I would get a B-scan preoperatively to evaluate choroidal thickness.
My thoughts are as follows: I would go with Option 1 here. After enlarging the pupil and staining the capsule, I would inject dispersive OVD—or, if necessary to deepen the AC, a viscoadaptive OVD. Option 3 is reasonable, but I am not sure how helpful mannitol would be for an eye that is already soft. If the chamber does not deepen and the axial length is normal, a vitreous tap could be performed. I would avoid this for eyes with an axial length less than 20 mm due to the uncertain size of the pars plana.
I would make a scleral window in two types of eyes: 1) those with an axial length less than 20 mm in which the chamber cannot be maintained and 2) those in which the chamber can be formed initially but then shallows during the case. The latter suggests that there is an intraoperative choroidal effusion.
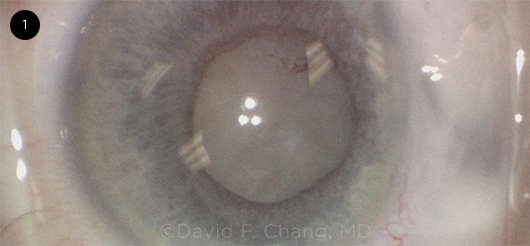 |
|
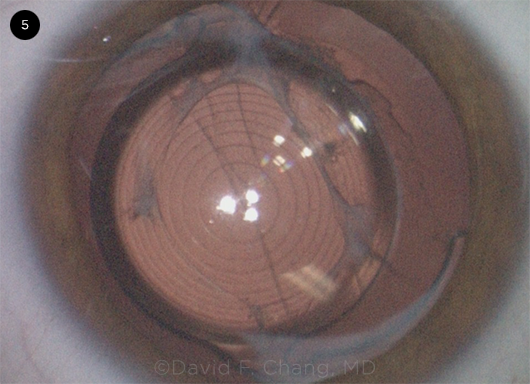
CASE 1. This image shows a white lens with a fixed small pupil and extremely shallow AC. The patient had a prior peripheral iridotomy for narrow angles and a trabeculectomy.
|
Case 2: Traumatic Zonulopathy
This patient was referred for an advanced cataract with a prior history of a Nerf dart injury to the eye.
Q2.1 Phacodonesis is noted during completion of the capsulorrhexis. How would you proceed?
| Commence careful phaco |
24%
|
| Insert a capsular tension ring (CTR) prior to starting phaco |
12%
|
|
Insert capsular retractors prior to starting phaco
|
44%
|
|
All of the above
|
18%
|
|
Convert to large-incision manual extracapsular cataract extraction (ECCE)
|
2%
|
John Berdahl When significant phacodonesis is observed during cataract surgery, the most important next step is to stabilize the lens capsule/zonular complex. The best way to accomplish that is to insert capsular retractors. It is important to use capsular retractors (versus iris hooks) because they extend all the way to the equator of the lens capsule. Additionally, they distribute the forces across the length of the retractor as opposed to just at the tip, which could lead to a radial tear in the setting of using an iris hook to retract the capsule. Usually a minimum of three capsular retractors is necessary unless there is only a very localized area of zonular loss. I typically prefer to have four retractors in place. Often, as the lens removal continues, an increasing amount of zonular weakness becomes apparent.
Once the cataract is adequately removed, a CTR can be placed to prevent centralization of the peripheral capsule. A critical moment occurs when determining if the capsule is stable enough to place the IOL. It can be difficult to tell if the capsule/zonular complex is stable enough because the capsular support may provide a false sense of security. However, if after lens insertion, it is determined that the complex is not stable enough, a retractor can be used to restabilize the bag. A sutured capsular tension segment (CTS) also may be used.
CASE 2 UPDATE: The capsular bag is preserved despite severe circumferential zonulopathy.
Q2.2 Which IOL and fixation method would you choose?
| IOL in bag without a CTR |
14.5%
|
| IOL in bag with a CTR |
32.7%
|
|
IOL in bag following a sutured CTR or CTS
|
14.5%
|
|
Three-piece IOL in sulcus
|
34.5%
|
|
Scleral fixation of a posterior chamber (PC) IOL
|
3.6%
|
|
Other method
|
0%
|
Brandon Ayers In this case, we have a cataract with significant zonulopathy and preservation of the capsular bag. Reviewing the audience response shows that about two-thirds of the audience would place the lens either in the sulcus or in the bag after placement of a CTR. The final third would either place the lens in the bag without additional support systems, suture the IOL, or use a sutured CTR or CTS.
As a referral anterior segment surgeon, I’m forced to deal with zonulopathy on a daily basis. I firmly believe that if you can use the capsular bag, it is best to do so. In many cases, the capsular instability is regional and limited to three or four clock-hours or less. In this situation, a CTR is often enough to stabilize the situation and allow placement of the intended PC lens in the bag.
In cases of more severe zonulopathy such as PEX, homocystinuria, or severe trauma, a CTR may not be enough to allow safe and secure placement of an IOL in the capsular bag. In these cases a suitable CTR or CTS can be used to help stabilize the capsular bag. Once the bag is stabilized, a single-piece or three-piece implant can be placed in the bag. In this scenario, a three-piece IOL can also be placed in the sulcus. It is my preference to fixate a sulcus lens—I either capture it in the capsulorrhexis or suture the haptics to the iris. In many cases, a nonfixated sulcus lens will rotate and eventually find an area of weakness allowing subluxation.
If the zonulopathy is so severe that the surgeon feels the capsular bag cannot be used, an alternative form of IOL placement will need to be considered. In the majority of these cases a vitrectomy will need to be performed; after this, the implant can be placed. The IOL can be fixated in the AC or scleral fixated using suture or haptic fixation, depending on the surgeon’s comfort level with each technique. In some cases, the surgery will need to be staged in order to operate alongside a vitreoretinal surgeon. This will help make sure the vitreous is adequately removed prior to placement of the lens implant.
CASE 2 UPDATE: After a three-piece monofocal IOL is implanted in the sulcus, there is vitreous prolapsing into the clear corneal incision.
Q2.3 How will you manage vitreous strands noted following IOL implantation?
| Sweep vitreous from incision with a spatula |
2%
|
| Use a Weck-Cel sponge and scissors; then sweep with a spatula |
4.1%
|
|
Perform anterior vitrectomy via the phaco incision
|
12.2%
|
|
Perform anterior vitrectomy via a limbal side-port incision
|
49%
|
|
Perform anterior vitrectomy via a pars plana sclerotomy
|
32.7%
|
Thomas Kohnen In this case, almost half of the respondents elected for an anterior vitrectomy via a limbal side-port incision with a mechanical cutter. This would also be my choice. However, when there is vitreous in the AC (which could be tested with the injection of vitreous-staining triamcinolone in the AC or with an air bubble injection through a side-port incision followed by a spatula sweep), testing the main wound and the paracentesis with a sponge and cutting the vitreous outside with scissors can be an important first step. This would then be followed with anterior vitrectomy via two limbal side-port incisions—and I would use a separate irrigation and anterior vitrectomy/aspiration approach through these incisions. In other words, the cutter and irrigation are separated via the two paracenteses.
This strategy has the main advantage of cutting the vitreous where it appears and not provoking more vitreous out of the eye through the main incision. I abandoned machine vitrectomy through the main wound many years ago because of this reason. If continuous flow of vitreous occurs with this anterior vitrectomy approach, I would risk a pars plana vitrectomy after having installed a trocar through the pars plana. In this instance, one could also withdraw the irrigation of the bimanual irrigation system through a paracentesis. The vitreous would be pulled back from the AC into the anterior part of the vitreous cavity and would not continuously extract into the AC.
Q2.4 In general, when would you implant a CTR for a patient with pseudoexfoliation?
| Only if significant zonulopathy was noted during surgery |
37.3%
|
| If any zonulopathy was noted |
43.3%
|
|
With a premium IOL, even if no zonulopathy was noted
|
0%
|
|
All cases, even if no zonulopathy was noted
|
4.5%
|
|
I don’t use or have experience implanting a CTR
|
14.9%
|
Rich Hoffman None of the responses is incorrect except, perhaps, Option 5.
The fact that 15% of respondents “do not use or have experience implanting a CTR” is unacceptable. Every cataract surgeon should be familiar with CTRs and their indications. The judicious use of a CTR during a difficult and challenging case of PEX (or any case with zonulopathy) could make the difference between a successful operation and a complete disaster.
I would encourage those surgeons who are unfamiliar with CTRs to practice implanting them on normal eyes so that they can be familiar with their use when needed for zonulopathy cases. There is really no harm implanting CTRs in normal eyes, and I commonly use these in long eyes implanted with toric IOLs to help assist in rotational stability.
Although 37% of respondents would use CTRs only in cases of significant zonulopathy, 43% would use them for any amount of zonulopathy. In general, if the zonulopathy is four clock-hours or less, a CTR is recommended to help stabilize the capsular bag. Zonular degradation is a progressive condition in patients with PEX, so placing a CTR in patients with any degree of zonulopathy can only help. Placing a CTR in PEX eyes does not guarantee prevention of capsule phimosis or IOL/bag subluxation, but it may help with IOL centration intraoperatively—and, perhaps, until late IOL/bag subluxation develops. (The latter usually takes place in a small percentage of PEX patients, typically seven to eight years following surgery.)
Like 4.5% of respondents, I personally place CTRs in all of my PEX patients. My reason for doing this is controversial. I have found that approximately 5% of these patients develop late IOL/bag subluxation, and the presence of a CTR in the bag helps facilitate repair of these subluxations. With a CTR in place, the location of the haptics is not important, as there are now 360 degrees of bag equator to fixate to the sclera with either 9-0 Prolene or CV-8 Gore-Tex sutures. This is helpful in eyes that do not dilate well, such as those with PEX. In addition, single-piece IOLs are more challenging to fixate to the sclera than three-piece IOLs when there is no CTR in place. Yes, it can be done, but there is a slightly greater chance of the suture cheesewiring around the single-piece haptic. This may be especially more likely when the IOL is subluxed inferiorly and the haptics are oriented horizontally. With a CTR in place, the importance of the IOL’s design and the orientation of the haptics are moot. A CTR in the bag simplifies the planning of the fixation procedure.
I receive referrals for subluxated IOL/bag complexes frequently, and I am always relieved when I know a CTR is in the bag—and always slightly frustrated when it is not. If you practice long enough, you will see these subluxations in your own PEX patients, sometimes after minor trauma. They can be easily repaired . . . and this is easier if a CTR was implanted at the time of the initial operation.
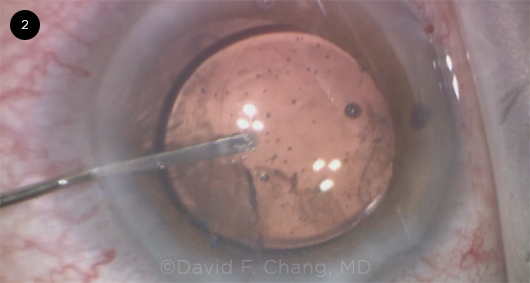 |
|
CASE 2. This eye has a history of blunt trauma. Due to severe zonulopathy and phacodonesis, a three-piece monofocal IOL was implanted in the sulcus. At this point, sweeping the peripheral AC revealed vitreous incarceration in the clear corneal incision.
|
Case 3: Rock-Hard Cataract
This 79-year-old patient presented with bilateral advanced cataracts and has counting fingers (CF) vision in both eyes. She was convinced to undergo surgery first in the eye with a less brunescent lens, and this was successful. She now has scheduled surgery in the eye with the rock-hard cataract.
Q3.1 What is your preferred technique for a rock-hard nucleus?
| Divide and conquer phaco |
34.2%
|
| Phaco chop |
25.3%
|
|
Prechop (e.g., miLOOP)
|
15.2%
|
|
FLACS
|
5.1%
|
|
Manual ECCE
|
20.3%
|
Steve Arshinoff The majority of the audience (59.5%) would elect to perform either phaco chop or divide and conquer for a rock-hard cataract. These are very reasonable responses and are consistent with cataract surgical teaching over the past three decades.
I have always taught that you can do phaco in any case in which the phaco tip can enter the nucleus, as long as you chose appropriate phaco parameters and a suitable OVD. Lower flow rates (<25 cc/min) are preferred as they allow the OVD to stay in the eye longer to protect the endothelium.
With both phaco chop and divide and conquer, the phaco tip enters the nucleus, one sculpting deeply and the other burying deeply to support the nucleus by the phaco tip to enable chopping. It is important to use pulse phaco in these cases to reduce chatter and to avoid heating the wound, which could cause a wound burn. It also is important to use an appropriate OVD strategy. My favorite is the Tri–Soft-Shell Technique: The dispersive is injected first to protect the endothelial cells, followed by a viscoadaptive to deepen and stabilize the AC, and, finally, by a thin layer of BSS—or, in very dense cases, trypan blue. This final “watery layer” enables the surgeon to create the capsulorrhexis against much less resistance in a chamber deepened and stabilized by the viscoadaptive. My own preference—to chop but divide and conquer in dense nuclei—is also an excellent strategy. With both methods, the surgeon should attempt to work at the level of the capsulorrhexis or in the nucleus and should try to avoid phacoing near the cornea. All lens fragments should be left in the eye to facilitate nuclear rotation until all the pieces have been created, prior to removing any. Once the pieces have all been chopped it is advantageous to change from the chopper to a Koch style lens rotator, which facilitates rotation and is safer for the posterior capsule.
Unlike 20.3% of the audience, I would reserve manual ECCE for cases in which the phaco tip cannot enter the nucleus because of its extreme density. Once the surgeon moves to ECCE, attempts to control postoperative astigmatism have essentially been abandoned. Patience with the phaco usually makes moving to ECCE unnecessary.
Prechopping with miLOOP (Zeiss) or with an Akahoshi prechopper is an option chosen by 15.2% of the audience. However, in a very dense lens, it is difficult to see where the miLOOP is being positioned, and Akahoshi prechoppers tend to exert a lot of stress on the capsule in these lenses.
Currently, I advise all patients with dense cataracts to undergo FLACS. This was not a popular choice among respondents (5.1%) to the question, reflecting the limited use of femto by many surgeons. However, my experience is that the laser (I use Johnson & Johnson’s Catalys system) can convert the challenging removal of a very dense cataract into a simple surgery—similar to the removal of a moderate cataract with appropriate settings—and it will allow you to phaco the cases you were considering for ECCE. As long as the femto OCT can see most of the lens depth, and it can usually see it all, the cases are much easier and have far fewer risks of complications. As time goes on, as with all other new technology, we are learning that the femtosecond laser may not provide much advantage for a regular case, but it does provide huge benefits in some cases, particularly in instances of very dense cataracts, PEX, and posterior polar cataracts.
Q3.2 Phacodonesis is noted during the capsulotomy. What would you do next?
| Commence phaco carefully |
17.7%
|
| Implant a CTR prior to starting phaco |
6.5%
|
|
Implant capsular retractors prior to starting phaco
|
48.4%
|
|
All of the above
|
9.7%
|
|
Convert to manual ECCE
|
17.7%
|
Uday Devgan and Bill Wiley For this case of a rock-hard cataract and very weak zonular support, we know that it will require much more time and ultrasonic energy to disassemble and aspirate the nucleus. This poses a high risk for capsule damage, vitreous prolapse, and retained nuclear pieces. While the audience decided to proceed with phaco with the use of capsular retractors or a CTR, we chose converting to manual ECCE. This allows quick and safe removal of the nucleus without creating additional capsular stress and with the added benefit of preserving corneal endothelial cells. The patient should be given additional local anesthesia in the form of a retrobulbar, peribulbar, or sub-Tenon block.
Indeed, in this case, the surgery was converted to manual nucleus extraction. Cataract surgeons in the United States rarely perform ECCE surgery and may not be aware that the procedure has evolved tremendously over the years. The preferred technique that we teach our residents is MSICS: manual small-incision cataract surgery, a much-improved version that creates an incision that seals with minimal or no sutures. This is often the preferred technique for the very dense cataracts encountered on charity surgery mission trips in many countries. David Chang published a study that compared phaco in his hands to MSICS by another surgeon when operating on similarly dense cataracts. The results showed that MSICS actually provided better results and a faster recovery than phaco.1
The key step for MSICS is to create a shelved scleral incision that is funnel shaped and can be secured with a single stitch. The initial temporal corneal phaco incision should be abandoned, and the surgeon should move to the superior sclera to make the MSICS incision. There are many good videos online2 demonstrating the MSICS technique, and we encourage all cataract surgeons to spend a moment learning it.
___________________________
1 Venkatesh R et al. J Cataract Refract Surg. 2010;36(11):1849-1854.
2 https://cataractcoach.com/?s=msics. Accessed Dec. 21, 2021.
Q3.3 What IOL would you implant in this case after a manual ECCE with an intact PC?
| Single-piece foldable acrylic IOL |
6.8%
|
| Three-piece foldable acrylic IOL |
73%
|
|
Three-piece PMMA IOL
|
12.2%
|
|
Single-piece PMMA IOL
|
8.1%
|
|
Other IOL
|
0%
|
Bonnie Henderson The advent of the foldable IOL was celebrated because the wound size could be decreased to half of that created with manual ECCE. But in this case, it is interesting that 73% of the respondents chose a three-piece foldable IOL even in the presence of a larger (6- to 7-mm) incision. I assume this was because most surgeons have foldable IOLs readily available on consignment and do not have PMMA IOLs on hand. It would be interesting to know whether audience members would still choose to implant a foldable IOL if they also had nonfoldable IOLs available.
Most of the respondents chose a three-piece over a single-piece IOL. I agree with this choice because the haptics of a single-piece IOL can occasionally become anteriorly displaced, and a three-piece IOL is less likely to cause inflammation by chafing of the posterior iris. Additionally, the haptics of three-piece IOLs tend to be stiffer than those of the soft one-piece foldable acrylic IOLs. This stiffness may provide additional stability to the capsular bag to prevent contraction and subsequent decentration.
A single-piece foldable acrylic IOL may have been chosen by some respondents because the implantation is often gentler than that which occurs with a three-piece lens. For instance, the single-piece foldable acrylic haptics unfold more slowly. Whenever there is zonular or capsular compromise, IOL delivery becomes even more important to avoid further capsular damage. Thus, a single-piece foldable acrylic can offer a more controlled option. However, in this case, phacodonesis was noted. If the IOL/capsular bag complex becomes unstable and the IOL is a three-piece one, it can be fixated to the sclera or iris by several different techniques. If the IOL is a single-piece foldable acrylic, the options for fixation become limited. Specifically, single-piece foldable acrylic IOLs cannot be used in the Yamane technique, placed in a scleral groove for a “glued” technique, or iris-fixated due to the lack of angulation between the optic and haptics.
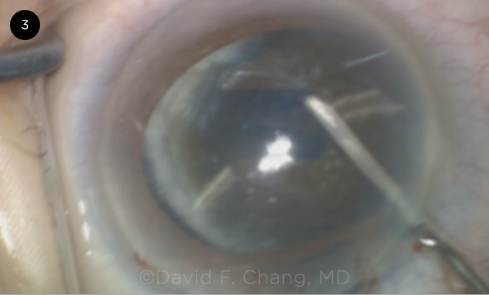 |
|
CASE 3. Zonulopathy became evident during the capsulotomy with lateral displacement of the lens in this eye with an advanced brunescent cataract.
|
Case 4: Rock-Hard Cataract + PCR
This patient’s left eye is blind due to complications of a retinal detachment (RD) that occurred following cataract surgery in that eye. Despite worsening acuity, she is hesitant to have surgery in her “good” eye due to fear of going blind if any complications occur.
Q4.1 What would you advise for her second eye in this scenario? She has 20/50 vision and a brunescent 4+ nuclear cataract.
| Delay surgery for as long as the patient can function |
7.8%
|
| Wait until the visual acuity (VA) worsens a few more lines |
6.3%
|
|
Suggest that doing surgery now is a reasonable option
|
50%
|
|
Encourage doing surgery now
|
28.1%
|
|
Encourage doing surgery and refer the patient to another surgeon
|
7.8%
|
Paul Singh I would encourage the patient to have the surgery done soon, and it looks like the audience also agrees that surgery is a good option for this patient.
The patient’s hesitation is understandable: No doubt the outcome in the first eye is impacting her willingness to proceed with surgery in the other eye. These patients also often do not appreciate the amount of decline in vision since there is no vision in the other eye to compare with. She likely already has poor depth perception; thus, any slight worsening of the cataract could cause her significant disruption in her daily functioning and, therefore, could result in serious risks to her life.
In these situations, it is important to take the time to help educate the patient on the increased risk of complications inherent in waiting longer. We also need to establish a strong doctor-patient relationship. Education provides a great opportunity to build or reinforce trust with the patient. Actively including the patient’s family members in the decision-making process is extremely important as well. I tell patients there is a risk with any surgery, but the risk of cataract surgery does not decrease—rather, it increases with time as the cataract becomes harder and denser. I would also tell this patient that the longer we wait, the more energy we need to use to remove the cataract, thus the higher risk of swelling of the cornea and a longer recovery postoperatively. Since she has only one seeing eye, early post-op vision is important for her daily functioning; therefore, the earlier we remove the lens, the better chance we have of providing good useful vision on post-op day 1. Being open and honest about the surgical challenges is a must, but providing context is also as important. I often say, once we take out the cataract, we are normally done with the cataract forever, so there is no advantage to waiting.
I do offer and suggest FLACS in these situations, as the femtosecond laser will likely help decrease the amount of phaco energy. In addition, it should provide a nice round, strong, centered capsulotomy—and in case there were issues during surgery, this may be beneficial if we need to optic capture a sulcus lens, for instance. If one doesn’t have access to a femtosecond laser, then using devices such as the miLOOP can help decrease energy and allow for less stress on the zonules.
CASE 4 UPDATE: This particular patient was actually CF in her phakic eye and underwent phaco. However, a PC rent is noted during nuclear emulsification.
Q4.2 After noting posterior capsule rupture (PCR) with multiple nuclear fragments still present, how would you proceed?
| Continue performing “slow motion” phaco |
28.8%
|
| Extract the nuclear fragments with a lens loop prior to an anterior vitrectomy |
21.9%
|
|
Perform an anterior coaxial vitrectomy through the phaco incision
|
9.6%
|
|
Perform an anterior biaxial vitrectomy through a separate limbal incision
|
23.3%
|
|
Perform an anterior biaxial vitrectomy through a pars plana sclerotomy
|
12.3%
|
|
Abort surgery and refer the patient
|
4.1%
|
Keith Warren The recommended first order of business is to inspect the surgical field and vitreous status. Assessing the size, number, location, and type of lenticular fragments, the capsule status, and the extent of vitreous loss is paramount in planning and executing a successful outcome.
After placing a viscodispersive at the phaco tip prior to fragment removal, the next step is to assess the vitreous status. Further attempts at removing any residual lens fragments should be delayed until any vitreous loss or prolapse has been addressed, contrary to the most popular response to this question. Attempts at removing fragments prior to addressing any vitreous loss is likely to result in removal of more than just lens fragments, and further complications are likely to follow. The lens fragments can be compartmentalized with a viscodispersive agent, and the vitreous should then be “stained” with washed triamcinolone or Triesence (Alcon). The phaco incision should be secured with a suture—and, as correctly suggested by the second most popular response, any vitreous that is identified should be removed by anterior biaxial vitrectomy through a separate limbal incision away from the primary wound. Vitrectomy should be carried out from the periphery toward the pupillary margin to avoid traction at the vitreous base. While a biaxial anterior vitrectomy via a pars plana approach can also be effective, it should not be considered as the primary approach; instead, it should be reserved for the experienced surgeon, as the risk of retinal complications is higher.
Once the vitreous has been excised from the AC, the residual lens fragments can be removed via phaco or lens loop, whichever the surgeon finds most comfortable and effective. With adequate capsular support, a three-piece lens should be placed into the ciliary sulcus and, if possible, captured in the capsulorrhexis. Minimal intraoperative iris manipulation and generous use of corticosteroids and NSAIDs are recommended to reduce the degree of postoperative inflammation. Referral for vitreoretinal evaluation is strongly recommended for assessment of the macula status and a careful examination of the vitreous base to rule out any significant peripheral pathology that warrants treatment.
Q4.3 How would you extract the remaining nuclear fragments after performing an anterior vitrectomy?
| Resume phaco |
4.5%
|
| Insert an IOL as a scaffold prior to resuming phaco |
77.6%
|
|
Insert a Sheets glide as a scaffold prior to resuming phaco
|
10.4%
|
|
Extract the fragments with a lens loop
|
6%
|
|
Leave the nuclear fragments and refer to a vitreoretinal surgeon
|
1.5%
|
Rudy Nuijts Resuming phaco to extract the remaining nuclear fragments after the anterior vitrectomy carries the inherent risks of pushing the fragments into the vitreous cavity due to positive pressure. Of course referral to a vitreoretinal surgeon is an excellent option if the cataract surgeon has little experience managing these types of cases: Better safe than sorry is not a bad motto!
Extracting the fragments with a Sheets glide or irrigating vectis is possible, but when using a Knoll-Pearce Irrigating Vectis, the fluid stream may force the fragments into the posterior vitreous. Compartmental separation with abundant OVD is key to avoid this. Clearly, the insertion of an IOL to block access to the PC (e.g., the scaffold technique) is the most secure method to keep nuclear fragments from dropping.
In terms of technique, a three-piece foldable IOL should be inserted beneath the nuclear fragments, and the leading haptic should be positioned above the iris or above the capsulorrhexis margin. Depending on the integrity of the capsulorrhexis, the IOL can be placed in the AC. This prevents the IOL from accidentally dropping into the vitreous. Given the density of the cataract, phacoemulsification of the remaining nuclear remnants should be performed cautiously with maximal protection of the corneal endothelium. The IOL is then dialed into the sulcus above the intact capsulorrhexis margin, and optic capture may be performed. Finally, do not attempt to perform an IOL scaffold in the sulcus if the capsulorrhexis margin is not intact.
Q4.4 For the IOL scaffold technique, where would you position the IOL?
| Place both haptics in the AC angle |
14.3%
|
| Place both haptics in the ciliary sulcus |
65.3%
|
|
Place one haptic in the AC and leave one externalized through the phaco incision
|
6.1%
|
|
Place one haptic in the ciliary sulcus and leave one externalized through the phaco incision
|
10.2%
|
|
I would not use this technique
|
4.1%
|
Ashvin Agarwal Options 1-4 would allow the surgeon to perform a good IOL scaffold technique. Option 2, in which the IOL is in the ciliary sulcus, would give the surgeon the best opportunity for emulsifying the nucleus while providing the maximum space in the AC, which would protect the endothelial cells. I would definitely choose this as my first option, hands down.
Option 1, in which the IOL is in the AC angle, does give the surgeon some freedom to maneuver the haptics into the sulcus—sometimes, the nucleus is large and the pupil is small. Options 3 and 4, in which one haptic extends out of the phaco incision, are most definitely needed at times, as it gets tricky if the pupil is large and the IOL might drop during the IOL scaffold emulsification. I would add that if the nucleus is hard brown/black grade 4 or above, do not try to emulsify it in the AC using a phaco probe. Instead, I would urge the surgeon to learn a small-incision technique and express this hard nucleus to protect the corneal endothelium.
As for the last choice: This is never an option!
CASE 4 UPDATE: After an anterior vitrectomy, a three-piece monofocal IOL is implanted into the ciliary sulcus beneath the multiple nuclear fragments. The remaining fragments are successfully emulsified with phaco, and the IOL is left in the sulcus with optic–capsulorrhexis capture.
Q4.5 Would you inform the patient of a “complication” on post-op day 1?
| No, because of the excellent anatomic result |
1.4%
|
| Yes. But I wouldn’t refer to a retina specialist if no problems arose |
35.6%
|
|
Yes, and I would automatically refer to retina specialist during the first two weeks postoperatively
|
57.5%
|
|
Yes, and I would automatically refer to retina specialist later in the post-op period
|
5.5%
|
|
I would first consult with my malpractice insurer before discussing the situation with the patient
|
0%
|
Zaina Al-Mohtaseb Absolutely yes! It is our responsibility as physicians to inform patients of complications that occur during surgery.
This particular case involves a monocular patient with a history of an RD in the other eye who was hesitant to have surgery in the first place.
Discussing surgical complications after such a case is always much easier if the possible complications were discussed during the preoperative consent process. In this instance, I would tell the patient that she had a dense cataract and prior complications. Before surgery, I would spend time discussing what could happen. In the recovery area, I would sit with the patient and any accompanying family member and let them know about the problem I encountered during surgery. I would reassure them that we would discuss this in more detail and that I would have a plan and would be with them the whole way. I would call the patient to check on her that night and would make sure I saw her as soon as she arrived the next morning. I would tell her again what happened and how happy I was to be able to remove all the lens materials and put in an IOL—and I would explain that risks of endophthalmitis and retinal tears/detachments increase with a PC tear, especially in a patient with a history of RD in the other eye.
It is important to avoid causing traction on the vitreous during the procedure, but I would definitely send this patient to see a retina colleague postoperatively for evaluation in the early post-op period. I would make it easy for her by calling my retina colleague and having my staff make the appointment. Finally, I would continue to check on the patient in both the short- and long-term.
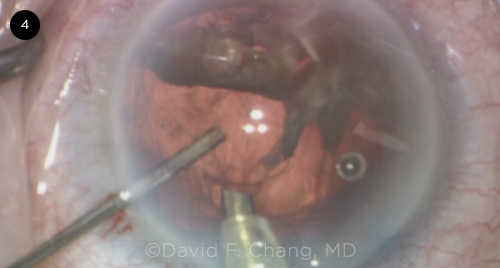 |
|
CASE 4. In this eye with an ultrabrunescent rock-hard cataract, a PC defect was noted during phacoemulsification.
|
 |
|
KELMAN AWARD. Michael E. Snyder, MD (center), with Drs. Fram (left) and Chang (right). Dr. Snyder presented the 16th Annual Charles Kelman Lecture.
|
Case 5: Young Patient Hates Glasses
This 35-year-old patient with bilateral fetal nuclear cataracts has low myopia and astigmatism. He doesn’t want to wear glasses but demands excellent vision at night. He has obsessive-compulsive disorder and depression.
Q5.1 Which IOL would you recommend for this young patient with natural myopic astigmatism?
| Monofocal IOL (targeting plano) |
5.6%
|
| Monofocal IOL (targeting slight myopia) |
18.5%
|
|
An extended-depth-of-focus (EDOF) IOL
|
38.9%
|
|
Multifocal IOL (e.g., trifocal)
|
9.3%
|
|
Other IOL
|
0%
|
|
I would refer this patient elsewhere
|
27.8%
|
John Vukich The first thing I would do is try to set realistic expectations. I would explain to the patient that nothing will ever be as good as a natural human lens in perfect working order. This is not the case for this patient. He has to understand that he has needed glasses his whole life and has cataracts and astigmatism. As a surgeon I will do my best, but patients such as this one have to accept that they may still need glasses for some activities.
In young patients the profound pseudophakic presbyopia that occurs with bilateral single vision IOLs for distance is very dissatisfying. Monovision with a toric IOL is a good option, and the key to success is to ensure that the distance eye is as close to plano as possible. The most common audience response was to use an EDOF IOL. I agree this is a reasonable option, but it is important to be certain that this patient understands that there may be slight halos at night and that he may still need low-power reading glasses. The second most common answer would be to wisely refer this patient elsewhere. If I saw this patient, I would use a Light Adjustable Lens (LAL; RxSight). The LAL provides the highest likelihood of best uncorrected distance vision and also allows the patient the opportunity to experience monovision, which can be adjusted prior to locking in the power.
CASE 5 UPDATE: He received a Symfony toric IOL (ZXT 300; Johnson & Johnson) but has 1.5 D of residual cylinder postoperatively.
Q5.2 How would you manage his toric IOL, which is 13 degrees misaligned?
| I would not recommend rotating/repositioning this IOL and would prescribe glasses for residual astigmatism |
2.9%
|
| PRK/LASIK enhancement for residual astigmatism |
13%
|
|
Perform astigmatic keratotomy for residual astigmatism
|
0%
|
|
Recommend surgical rotation of toric IOL
|
81.2%
|
|
Refer for surgical rotation of toric IOL
|
2.9%
|
Sumitra Khandelwal Rotation of toric lenses can be a challenge. Studies have found that the rotation can occur as early as one hour after surgery. Lee et al. reviewed the rotational stability of this exact lens with its monofocal counterpart and with a different toric presbyopia-correcting lens. The Symfony IOL was more likely to rotate and require repositioning than the ReStor IOL (Alcon) and the Tecnis monofocal (Johnson & Johnson).1
The first question I would ask any patient with a refractive error following surgery is how they feel about their vision. A small amount of residual astigmatism along with a small amount of myopia at times can provide extended depth of focus for patients. However, in this setting, I will assume he will be unhappy because of the amount of cylinder and his personality. Likely, the tech will let him know how much astigmatism he still has.
Option 1—just leaving it alone—is not a good option in patients who expect excellent outcomes. Option 2 may be a reasonable one if the patient has spherical as well as astigmatic refractive error, as one can correct both with precision. I also use this option if I recall the surgery had challenges such as sedation, patient movement, floppy iris, or zonular weakness. These are eyes I do not want to enter again. With regard to Option 3, this amount of cylinder is likely too high, but this option could be reasonable in cases involving lower amounts of astigmatism. Option 4 would be my treatment of choice, as it appears the toric has rotated since surgery.
I would run the Berdahl & Hardten Toric IOL Calculator to determine what the next steps are. If it recommended rotation, I would proceed with that strategy. The ideal time for rotation has been debated. Oshika et al. concluded that the optimal time of repositioning is one to three weeks after initial surgery.2 Rotating earlier than one week can result in re-rotation; rotating later may be more difficult due to bag-capsule contraction. My method is to place the paracentesis in a location that allows me to easily get where the haptics are located. I use a Palay cannula on viscoelastic to unzip the AC from the optic, and I push some viscoelastic posterior to the optic to dissect it off the PC. It is important to take one’s time on each step and not overinflate the AC. Dissecting the haptic can take more time (but less is needed with the Tecnis platform, which does not have the haptic bulb). Visualization is key, so if I cannot see the haptics and they appear to be stuck, I use iris hooks to dilate the pupil further. Once the IOL is freely mobile in the back, I rotate it into the position recommended by the calculator. I remove the elastic and let the AC shallow to allow the optic to adhere to the PC. A CTR can be placed if one thinks bag rotation is due to zonule weakness or a floppy large bag, although studies have not confirmed whether this helps.
___________________________
1 Lee BS et al. J Cataract Refrac Surg. 2021;47(5):622-626.
2 Oshika T et al. Ophthalmology. 2018;125(1):31-35.
CASE 5 UPDATE: Toric IOL surgical rotation improves the patient’s vision, but he complains bitterly about dysphotopsia at night and has had to stop driving.
Q5.3 What would you recommend for persistent EDOF IOL starbursts at four months postoperatively?
| Give more time for adaptation (offer a trial of a miotic such as pilocarpine) |
20%
|
| IOL exchange to nontoric monofocal IOL |
4.3%
|
|
IOL exchange to toric monofocal IOL
|
34.3%
|
|
Implant a toric monofocal in the second eye with myopic target, then reassess
|
8.6%
|
|
Refer elsewhere for IOL exchange
|
32.9%
|
Julie Schallhorn There are many reasons for patients to be unhappy after cataract surgery, and the key to improving their satisfaction is to determine the reason for the photic phenomena. Addressing this issue really starts before surgery—all of our currently available multifocal IOLs will induce some photic phenomena. These halos and starbursts are intrinsic to the design of diffractive lenses, and patients need to be warned about this before surgery. Not all patients will experience symptoms, but they need to understand that they can occur.
For patients who develop issues after surgery, a thorough exam and a listening ear are warranted. Starting with a careful manifest refraction and proceeding to a good exam as soon as the complaints are noted can help to elucidate the cause of the visual complaints. Clinicians can use a front to back approach, in which they evaluate the lids, tear film, epithelium, lens position, and centration, as well as PC opacities and the macula. Although PC opacification (PCO) can worsen photic phenomena such as glare, halos, and starburst, it is important to figure out when the photic phenomena started. Problems with quality of vision that start on post-op day 1 are likely not due to PCO and will likely not resolve with an Nd:YAG capsulotomy, but problems that start several weeks after surgery in the presence of worsening PCO likely will.
In this case of a young patient who was unhappy with the halos and starbursts after receiving a diffractive EDOF lens, all of the other potential causes of these symptoms were evaluated and ruled out, and it was evident that this patient was unhappy with visual quality provided by the EDOF lens. In patients with complaints due to the optical qualities of an IOL, a trial of a miotic before driving at night is warranted early in the postoperative course. However, this patient most likely needs an IOL exchange to a monofocal toric lens.
CASE 5 UPDATE: Although an IOL exchange is recommended at month 4 postoperatively, he obtains additional second opinions and finally returns five months later (month 9 post-op) requesting an IOL exchange for a monofocal toric IOL. However, he now has significant capsulorrhexis contraction. During surgery, the capsular bag is inflated, and the IOL is successfully mobilized. However, the capsulorrhexis diameter is much smaller than the IOL optic.
Q5.4 During the IOL exchange, the contracted capsulorrhexis complicates IOL extraction. What would you do next?
| Slowly pull the IOL through the capsulorrhexis causing it to stretch |
6.9%
|
| Bisect the lens within the bag using IOL scissors placed into the capsular bag |
41.4%
|
|
Obliquely incise one edge of the capsulorrhexis and use forceps to re-tear it by pulling the new flap
|
41.4%
|
|
Make radial capsulorrhexis-relaxing incisions
|
6.9%
|
|
Abort the attempt and leave the EDOF IOL
|
3.4%
|
Marjan Farid Most cases of primary IOL exchange within the first two to three months are pretty straightforward, and the IOL can be safely mobilized with viscoelastic and rotated out through the AC opening. Unfortunately, the hope is that over six to 12 months, the patient starts to neuroadapt to the multifocal optic and an IOL exchange may be avoided.
In this case, in which an IOL exchange has been delayed, capsular contraction and phimosis may occur, making it difficult to simply rotate the IOL out of the anterior opening. Generous use of a combination dispersive/cohesive viscoelastic to mobilize the IOL and free the haptics from any peripheral scarring should be done first. Then attention needs to be focused on enlarging the AC opening. Often the best way to accomplish this—without having a radializing tear—is to make an oblique incision on one edge of the capsule and use capsulorrhexis forceps to re-tear a larger continuous capsulotomy. This freshening up of the anterior capsulotomy should aid in rotating the IOL out of the capsular bag and into the AC, where it can then be cut with microincision scissors and removed through a small corneal incision. Bisection of the IOL within the bag is another way of pulling out two smaller pieces through the smaller AC opening. However, with this technique of working within a confined and scarred capsular bag, there is a higher chance of traumatizing the PC and placing undue tension on the zonules.
Q5.5 Following IOL exchange, what would you do about the contracted capsulorrhexis?
| Leave it alone |
14.5%
|
| Leave it alone but implant a CTR to prevent further contraction |
8.7%
|
|
Leave it alone and schedule YAG-relaxing incisions if needed
|
36.2%
|
|
Make radial relaxing incisions in the capsulorrhexis edge
|
2.9%
|
|
Obliquely incise one edge of the capsulorrhexis and use forceps to re-tear it by pulling the new flap
|
37.7%
|
Vance Thompson In this case, the surgeon dealt with the contracted capsulorrhexis and did not damage it by cutting the IOL in the capsular bag after generous viscoelastic protection and separation between the implant and the capsule. After extracting the cut IOL pieces and placing the folded implant through the contracted capsular opening, a decision should be made on how to deal with this contracted capsulotomy opening, because continued capsular contraction can lead to tilt and decentration of the implant. One could consider a CTR, but I also feel that if my implant position is stable and not in an abnormal position, I’m probably going to simply remove the viscoelastic and then see the patient postoperatively for YAG-relaxing incisions.
I would avoid making radial relaxing incisions in surgery because the capsule in these situations can be under impressive tension, and those incisions can extend and be very difficult to deal with and even extend and tear posteriorly. I thus am not a big fan of intraoperative relaxing incisions or obliquely incising one edge of the capsulorrhexis and trying to do a tear. This is a recipe for an unpredictable situation and complication. As a result, I would simply leave it alone in surgery, schedule post-op YAG-relaxing incisions, and follow closely just in case I needed to extend them a bit with more YAG laser.
 |
|
CASE 5. This young patient was intolerant of halos following implantation of a diffractive EDOF IOL. He returned nine months after the original surgery with capsulorrhexis contraction and requested an IOL exchange.
|
Case 6: Posterior Polar Cataract
This 52-year-old patient has a posterior polar cataract with 20/200 vision.
Q6.1 What hydrosteps would you perform for this posterior polar cataract?
| Both hydrodissection and hydrodelineation |
1.7%
|
| Viscodissection and hydrodelineation |
22.4%
|
|
Hydrodelineation only
|
56.9%
|
|
Viscodissection only
|
15.5%
|
|
No hydrosteps
|
3.4%
|
Amandeep Rai With modern surgical techniques and technologies, rates of PC rupture continue to drop. However, posterior polar cataracts present a unique challenge for cataract surgeons, as these cases may have a preexisting capsular defect or be at elevated risk for intraoperative PC rupture. Of all our intraoperative maneuvers during cataract surgery, cortical cleaving hydrodissection is the riskiest for PC compromise in these polar cataracts due to the high hydraulic forces. The majority of the audience (98.3%) voted to skip hydrodissection entirely, and I would agree.
In order to facilitate phacoemulsification, the majority of the audience (56%) preferred hydrodelineation alone. Overall, 81% of the audience would perform some form of hydrodelineation, either alone or combined with another technique. Because the posterior polar opacity is near the nodal point of the eye, these patients tend to present earlier in life for cataract surgery, and the endonucleus tends to be soft. Once the surgeon sees the “golden ring” form with careful hydrodelineation, they can be confident that the endonucleus is separated and cushioned by the epinucleus and cortex. Depending on the density of the endonucleus, it can be simply aspirated as a whole—or a quick chop technique can be employed to create two heminuclei that can be removed using a hemiflip technique. Both of these approaches avoid the need for nuclear rotation, thereby reducing stress on the posterior capsule.
If rotation is necessary, or to facilitate removal of epinucleus and cortex, viscodissection can be employed. I agree with the 22% of the audience in that I typically perform both hydrodelineation and viscodissection in these posterior polar cases; I use a dispersive viscoelastic to facilitate cleavage of the epinucleus and cortex from the PC. In the event of an open PC, the dispersive viscoelastic can tamponade the anterior vitreous. Ideally, viscodissection is performed at multiple clock-hours with only small amounts of OVD, so that the viscoelastic wave does not reach the posterior polar opacity of any weak points within the PC.
CASE 6 UPDATE: As the nuclear fragments are removed, lens debris behind the PC suggests that a PC rent has occurred.
Q6.2 How would you proceed after noticing lens debris just behind the PC?
| Carefully continue coaxial irrigation and aspiration (I&A) |
2.2%
|
| Lower the irrigation bottle before continuing coaxial I&A |
24.4%
|
|
Switch to biaxial I&A
|
26.7%
|
|
Inject triamcinolone and resume I&A if no vitreous prolapse is detected
|
42.2%
|
|
Perform a pars plana anterior vitrectomy before continuing cortical I&A
|
4.4%
|
Tom Oetting Lens debris behind the PC is common. This finding implies that either lens material sneaked past the zonules with an intact PC or, more problematically, there is a tear in the posterior capsule. However, in this case with a known posterior polar cataract, I think evoking the transzonular route for the small fragments is simply denial of a PC defect.
When you are performing I&A for residual cortical material and you have discovered a PC defect, I would suggest the following strategy, which is a combination of Options 2, 4, and 5:
First, add a viscous dispersive OVD (I like Alcon Viscoat) to stabilize the chamber. Then, remove the I&A unit from the AC and make an additional paracentesis or pars plana incision for the anterior vitrector (your preference). Perform bimanual anterior vitrectomy to the level of the PC. As always with anterior vitrectomy, lower the bottle height enough to keep the chamber formed—but without creating any leaks around the instruments.
Use either the anterior vitrector with the cutter off or a separate 23-gauge Simcoe cortex extractor to separate the cortical material from the anterior capsule. Next, bring the residual cortical material to the center and then turn the cutter on (slow cut rate to 200/min). At this point, you will likely find a central tear. Stain with dilute preservative-free Kenalog (I use dilute 1:5 Triesence) and remove any residual vitreous.
Hopefully you have kept the AC intact for our next question.
Q6.3 What IOL would you implant in the presence of a small tear in the PC?
| A nontoric single-piece acrylic IOL in the capsular bag |
14.3%
|
| A toric single-piece acrylic IOL in the capsular bag following a posterior capsulorrhexis |
30.4%
|
|
A toric single-piece acrylic IOL in the ciliary sulcus with reverse optic capture
|
12.5%
|
|
A three-piece foldable IOL in the ciliary sulcus with optic–capsulorrhexis capture
|
41.1%
|
|
Other choice
|
1.8%
|
Elizabeth Yeu For a patient for whom a toric IOL is warranted for mitigation of their refractive astigmatism, a PC compromise is a tough situation. I generally will not attempt to fix more than about 1 D with astigmatic corneal relaxing incisions because of the potential ensuing complications that can occur, including greater dry eye, glare, irregular astigmatism, irritation, wound gape, and focal ectasia.
The top answer for the IOL choice from the audience, at 41.1%, was a three-piece IOL in the ciliary sulcus with optic capsulorrhexis capture. Fixation of the optic with an optic capture is a safe and fantastic technique to perform. Ideally, the capsulotomy should be well-centered and sized about 4.5 to 5 mm circumferentially. The second highest response rate, at 30.4%, favored turning the posterior compromise into a complete posterior capsulorrhexis and placing the single-piece toric IOL in the capsular bag. This is also an excellent choice if the posterior rent or compromise is central enough that it can be incorporated into the posterior capsulorrhexis. A good dispersive viscoelastic tamponade and cushion are key to preventing vitreous prolapse during this step.
Last, if there is minimal to no lens fragment loss, and the PC compromise does not lend itself to a posterior capsulorrhexis, capsular bag placement of a single-piece toric IOL with a reverse optic capture (lifting the optic anterior to the anterior capsulotomy) using a haptic tuck technique is a very effective alternative method that I’ve employed successfully. It is important to correct the lens power and toric power for the sulcus placement, as the optic will functionally be in the sulcus. The optic will likely have a square edge, so watch out for posterior iris chafing and higher levels of postoperative inflammation. I will not consider this a surgical Plan B for a short axial hyperope because of the greater potential of uveitis-glaucoma-hyphema (UGH) syndrome, given the closer proximity of the optic edge to the posterior iris.
Q6.4 What is your experience with posterior capsulorrhexis?
| Have had success with this once or twice |
18.5%
|
| Have successfully performed this more than twice |
1.9%
|
|
Have attempted this without success
|
1.9%
|
|
have no experience but may try it
|
63%
|
|
I have no experience and wouldn’t try
|
14.8%
|
Dick Lindstrom The primary purpose of a posterior capsulorrhexis is to convert a PC tear that might split out and extend into one that is strong, stable, and robust to the forces required to implant an IOL into the capsular bag. In this case, conversion of an irregular jagged PC tear into a smooth-edged, continuous posterior capsulorrhexis allowed the safe implantation of a one-piece acrylic toric IOL into the capsular bag with rotation to the proper astigmatism meridian.
I believe this is a skill that can be learned by any anterior segment surgeon who performs cataract surgery regularly. However, only 20.4% of surgeons in the audience have had success with this maneuver. Fully 63% said they have no experience but are interested in learning, while 14.8% were fearful of even trying it. This represents a significant learning gap that can be remedied by viewing videos and practicing in a laboratory with animal or cadaver eyes.
The goal is to create a continuous opening in the PC like that done on nearly every case in the AC while retaining an intact anterior vitreous face. A dispersive viscoelastic placed through the inadvertent or purposeful PC opening can separate the capsule from the anterior hyaloid face. The maneuvers required in making a posterior capsulorrhexis are familiar and nearly the same as those utilized when creating a continuous tear anterior capsulectomy. A microforceps is helpful, but a standard Utrata forceps can be utilized. The posterior capsulorrhexis can be small, but it must be continuous with no tags or tears. If PC opacity occurs later, the aperture can be extended with a YAG laser months or years after the primary surgery. Posterior capsulorrhexis is a skill worth learning, and it will allow the cataract surgeon to utilize the preferred IOL they and their patient selected prior to surgery. In addition, in complex cases, it can be utilized for posterior optic capture after vitrectomy.
 |
|
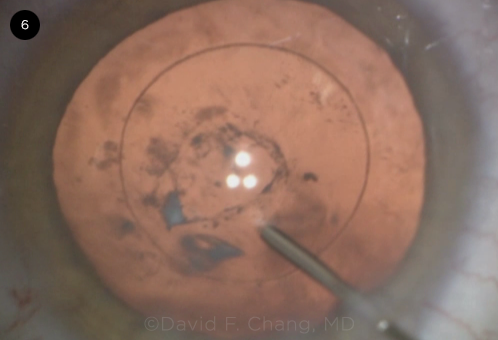
CASE 6. A Zepto capsulotomy was performed in this patient with a posterior polar cataract. A central PC defect was noted. The attached white posterior polar plaque can be seen just to the left of the cannula tip, with floating lens debris visible behind the capsule.
|
Case 7: Bag–Toric IOL Dislocation
This 52-year-old patient had bilateral T5 toric IOLs implanted six years ago. Although there was no PEX, a CTR was placed in each eye because of circumferential zonulopathy. He was referred back for a YAG capsulotomy in the right eye, but asymptomatic pseudophacodonesis was noted in the left.
Q7.1 What would you advise for this patient with pseudophacodonesis but good vision and a reasonably centered toric IOL?
| Observation only |
31.1%
|
| Suture-fixate the bag/IOL/CTR complex to the sclera |
24.4%
|
|
I’d normally observe, but I recommend Option 2 because of the high-power toric IOL
|
20%
|
|
IOL exchange now
|
0%
|
|
Refer this patient
|
24.4%
|
Kendall Donaldson This can be a very challenging situation, and the surgical approach will depend on both the clinical appearance as well as the patient’s symptoms at the time of presentation. Most patients with a standard nontoric monofocal lens would be less symptomatic than premium patients with toric or multifocal IOLs, due to quality of vision issues associated with decentration. This is a case that often involves education, making patients aware that their IOL is not positioned ideally, discussing the symptoms of further IOL displacement, and a discussion of the timing for surgery—including the risks and benefits of intervention versus observation. This would include the potential of IOL repositioning versus IOL exchange, as well as the potential for vitrectomy.
Many patients may be stable long-term in this situation. However, it is important to monitor closely, as this type of issue is much easier to address if the IOL is still in the anterior segment and has not fallen to the retina. This is particularly true with toric IOLs, which are difficult for retina specialists to orient appropriately and to fixate without support from a posterior approach. Thus, I would prefer to intervene a bit earlier with a toric or multifocal lens than with a standard monofocal. We also must consider that because we do not have three-piece toric IOLs available in the United States, if we are unable to save the bag/IOL complex, the alternative is lens exchange with a standard monofocal lens (with loss of the astigmatic correction).
When the patient returned three months following initial presentation, he was symptomatic, and the lens was clearly in the process of subluxation. Thus, the need for surgical intervention was more imminent. With further delay, the case could potentially lead to the inability to retain the toric IOL. The fact that the patient has a CTR in position makes the case easier as the IOL/capsular bag complex is more stable for fixation. In order to retain the IOL/capsular bag complex, suturing of the complex would be needed. Either Prolene or Gore-Tex suture material would be appropriate, depending on surgeon preference and experience.
CASE 7 UPDATE: The patient returned three months after the YAG capsulotomy with blur in his left eye and a partial sunset subluxation of the bag/IOL complex.
Q7.2 What would be your surgical approach for this sunsetting bag/toric IOL/CTR complex?
| Scleral suture–fixate the CTR with Prolene sutures |
14.9%
|
| Scleral suture–fixate the CTR with Gore-Tex sutures |
36.2%
|
|
Scleral suture–fixate the CTR with “belt loop” Prolene sutures
|
2.1%
|
|
Explant the IOL and CTR and exchange
|
0%
|
|
Refer this patient
|
46.8%
|
Yuri McKee and Cathleen McCabe The majority of respondents would refer out an in-the-bag toric IOL dislocation, which is the correct choice for a complex problem if the surgeon is not confident about using the required techniques.
The presence of a CTR in this case is helpful as this is an excellent anchor for the suture to fixate the entire IOL/capsule complex. Of those who are comfortable in repositioning the dislocated IOL in the proper axis, the majority chose Gore-Tex suture fixation. Gore-Tex suture material has been a trusted surgical tool for several years, although its use is off label for intraocular techniques. The important thing to remember with Gore-Tex is that the suture knots must be buried in the sclera to avoid future conjunctival erosion.
Fifteen percent of respondents chose fixation with Prolene sutures. We would remind them that 10-0 Prolene is not appropriate, given the known long-term risk of suture degradation. At a minimum, 9-0 Prolene should be used for scleral fixation—however, there are some concerns that this suture size may also be prone to long-term degradation. The minority of respondents were familiar with the new technique of using 6-0 Prolene in a belt-loop fashion to secure a dislocated IOL. Of course, 6-0 Prolene suture cannot be tied on the ocular surface—therefore, we use a heat-loop cautery to flange the end of the 6-0 suture as described by Sergio Canabrava from Brazil. Care must be taken to ensure the flange is buried within superficial layers of the sclera. Placing the flanged suture ends in a scleral pocket is one way of preventing conjunctival erosion over the flange.
We believe these new techniques with flanged sutures will become more popular in the coming years due to the longevity of larger-diameter suture material and the absence of knots that must be buried.
Q7.3 What is your preference for scleral suture fixation of an IOL?
| 10-0 Prolene (double armed) |
3.7%
|
| 9-0 Prolene (double armed) |
24.1%
|
|
Gore-Tex
|
20.4%
|
|
Intrascleral flanged Prolene sutures
|
0%
|
|
I don’t do these cases
|
51.9%
|
Steve Safran In this situation, in which an IOL within the capsular bag has dislocated due to zonular deficiency, and a CTR is within the bag, the simplest approach is to fixate the lens/bag complex to the sclera in its normal anatomic position. This is provided that the IOL is one we wish to keep, and the capsular bag is amenable to suturing (neither too flimsy nor too overburdened with Soemmering ring material).
I prefer to use CV-8 Gore-Tex for this application, as it is very secure and it appears to last very well, most likely for the patient’s lifetime. The knot is easy to completely bury within the sclera, and I prefer to place the suture itself at the base of a scleral groove so that it is below the surface of the eye and thus is unlikely to become exposed or to extrude.
It is quite easy to titrate the centration of the IOL/bag complex with Gore-Tex by adjusting the tension on the suture at each side. If one uses radial grooves there is virtually no risk of tilt, as there is no torquing of the lens/bag complex. If a CTR is present, one can lasso the CTR with or without the haptics in the suture, depending on how much fibrosis is present.
I prefer Gore-Tex over 10-0 and 9-0 Prolene, as those sutures are more likely to cheesewire and less likely to last over the long-term. A few years ago, before the technique was popularized, I tried using variations of a flange method with 6-0 Prolene. I abandoned this approach because I felt that it was not as easy to titrate or control centration of the lens/bag complex, and I was concerned that the flanges would extrude over time.
In the Yamane procedure, a tunnel is made, and the haptic flange can be buried within the tunnel, where it is held in place by tension from within the IOL. In contrast, when the lens/bag complex is lassoed with 5-0 or 6-0 Prolene, the needle goes straight in. Thus, no tunnel is created, and the flange needs to be big enough to secure the suture. In my experience, the mushroom flange tends to sit on the surface of the sclera. As there is no real tension on this suture, if the patient rubs the eye, the suture can extrude, leading to either a foreign-body sensation or erosion—and the latter raises the risk of endophthalmitis.
For this reason, I abandoned the use of 6-0 Prolene and went back to Gore-Tex about three years ago, as it had served me well in the past. It continues to be an excellent choice in my hands for these cases.
Q7.4 What is your preference for IOL fixation in the absence of any capsular support?
| Iris claw or AC IOL |
12.5%
|
| Iris suture fixation of PC IOL |
6.3%
|
|
Transscleral suture fixation of PC IOL
|
2.1%
|
|
Glued intrahaptic scleral fixation of PC IOL
|
4.2%
|
|
Yamane intrahaptic scleral fixation of PC IOL
|
37.5%
|
|
I would refer these patients
|
37.5%
|
Kevin Miller The audience was equally divided between the two popular choices, referring to someone with more experience and transscleral haptic fixation via the Yamane technique. Referral is always an appropriate option for anyone uncomfortable managing these difficult cases.
The Yamane technique is popular because it is quick and simple and does not require conjunctival or scleral dissection. Early complications of the technique are well known. They include lens tilt and decentration, iris chafe, vitreous hemorrhage, RD, haptic irritation of the conjunctiva and Tenon capsule, and haptic exposure in the tear film. Long-term complications are unknown because the technique is relatively new, but they will likely include haptic erosion through the conjunctiva. Iris claw lenses are not available in the United States for implantation into aphakic eyes. Inner scleral suture fixation was not a popular option, but it has a long and fairly safe track record. AC lens implantation is also a reasonable option for the older patient with a healthy corneal endothelium, as long as the lens is sized and positioned well.
The literature is inconclusive as to which approach to IOL fixation is best in the absence of capsular support. In the end, each ophthalmologist must decide what is best for the patient and what works best in his or her hands.
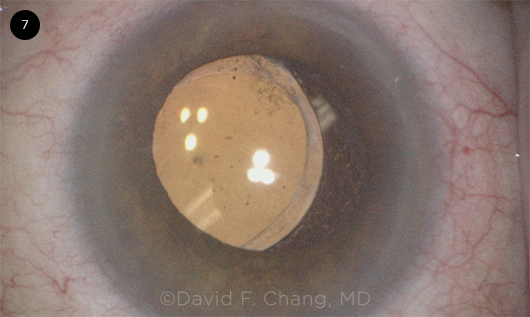 |
|
CASE 7. This symptomatic patient presented with a left sunsetting IOL/bag complex six years after a high-power toric IOL was implanted (surgeon seated temporally).
|
Case 8: Pseudophakic UGH Syndrome and a Sulcus IOL
This 76-year-old patient developed severe UGH syndrome after sulcus implantation of a single-piece acrylic IOL following PC rupture. He had severe glare from the iris transillumination defects.
Q8.1 How would you manage this single-piece acrylic IOL in the ciliary sulcus?
| Observe |
0%
|
| Suture-fixate IOL haptics to sclera (e.g., with Prolene) |
10.8%
|
|
IOL exchange—AC IOL or iris claw IOL
|
5.4%
|
|
IOL exchange—PC IOL
|
67.6%
|
|
Refer the patient
|
16.2%
|
Nick Mamalis Fortunately, 0% of the audience members stated that it was best to simply observe this patient. However, 10% stated that they would manage this by suture fixation of the IOL haptics to the sclera. The most important thing is that a combined 73% of respondents stated that they would treat this patient with IOL exchange, with either a PC IOL, an AC IOL, or an iris claw IOL. Moreover, 16.2% would refer this patient. This is very reassuring in that the vast majority of respondents understood that this IOL must be exchanged and that it is improper to leave a single-piece acrylic IOL in the ciliary sulcus.
The issue of single-piece acrylic IOL in the sulcus was initially raised during the Cataract Spotlight session at the 2008 Academy annual meeting. This eventually led to an extensive report regarding the placement of these IOLs that was published the next year.1 To this day, this report is the definitive work regarding complications of sulcus placement of single-piece acrylic IOLs, and it includes multiple recommendations on the proper characteristics of IOLs that are placed into the ciliary sulcus.
The problem with single-piece acrylic IOLs in the ciliary sulcus is that these lenses were specifically designed to be placed in the capsular bag. The single-piece haptics on these lenses are relatively thick and bulky, which subsequently allows contact with the posterior iris surface when they are placed into the ciliary sulcus. In addition, the optic edges are relatively square and rough, which can lead to chafing of the posterior iris surface. These issues can lead to pigment dispersion with subsequent iris transillumination defects and, eventually, to pigmentary glaucoma. The contact between the IOL and the posterior iris may also lead to breakdown of the blood-aqueous barrier, with chronic inflammation as well as recurrent microhyphemas leading to UGH syndrome. In addition, these lenses are too short to be placed into the ciliary sulcus. This could also lead to small amounts of decentration, leading to the sharp optic edge on these lenses scraping the posterior iris and, once again, causing issues with pigment dispersion.
It is reassuring that almost 75% of the respondents stated that IOL exchange was the proper way to manage a single-piece acrylic IOL in the ciliary sulcus.
___________________________
1 Chang DF et al. J Cataract Refract Surg. 2009;35(8):1445-1458.
Q8.2 What is your preferred method of PC IOL fixation with limited capsular support?
| Three-piece IOL in sulcus |
54.5%
|
| Sulcus implantation of a three-piece IOL with iris suture fixation |
3%
|
|
Sulcus implantation of a three-piece IOL with scleral suture fixation
|
21.2%
|
|
Intrascleral haptic fixation of a three-piece IOL
|
15.2%
|
|
Akreos IOL (Bausch + Lomb) with four-point suture fixation
|
6.1%
|
|
Other
|
0%
|
Amar Agarwal When patients do not have enough capsular support, one should check if the AC is intact. If so, as the audience has correctly voted, one can implant the IOL in the sulcus and do an optic capture also if needed. However, for cases in which the rhexis is not present, it is better to do a glued IOL or Yamane technique.
In performing a glued IOL procedure, it is important to remember the following key points, which can take your success to another level: 1) In large white-to-white measurement (>11 mm), perform a small peripheral iridectomy in the area of the scleral flaps. This way, you can do an anterior sclerotomy 0.5 to 1 mm from the limbus, which will give you more haptic to externalize. The iridectomy prevents any iris damage during an anterior approach, as the needle and glued IOL forceps passes through the iridectomy. 2) When you approach anteriorly, you may have an optic capture. Thus, you should learn the single-pass four-throw pupilloplasty technique, which will solve that issue immediately.
CASE 8 UPDATE: This patient later underwent an RD repair with a gas bubble. He subsequently developed opacification of the hydrophilic Akreos IOL optic and reduced vision.
Q8.3 How would you manage this opacified IOL?
| Observe longer; surgically remove it only if the patient can’t adapt |
43.3%
|
| Advise against further IOL exchange, as the patient has glaucoma |
3.3%
|
|
Perform an IOL exchange
|
33.3%
|
|
Refer elsewhere for IOL exchange
|
20%
|
Naveen Rao In this case, there was a macula-on RD, followed by intraocular gas placement during RD repair. This resulted in subsequent calcification of the hydrophilic acrylic Akreos AO60 IOL and reduction of the patient’s best-corrected VA from 20/50 to 20/200, which was attributed to the opacified IOL.
In general, I agree with the audience that surgery should be reserved for those situations in which the patient is truly bothered, unless there is risk of harm from observation alone. We must also keep in mind that this eye has the other significant problem of iris transillumination defects, which must also be dealt with to restore good visual function and to treat his severe glare. If the fellow eye had good vision—and if the patient wanted to avoid additional surgeries that could carry additional risks such as another RD or corneal decompensation—it would be reasonable to recommend observation. However, if the patient was very symptomatic, and if he accepted the possibility of needing additional surgeries in the future, then I would offer IOL exchange. My personal preference would be to exchange the Akreos AO60 for a Zeiss CT Lucia 602, which has a hydrophobic acrylic optic and therefore is unlikely to opacify even if he needed subsequent eye surgeries involving an intraocular gas bubble. I would secure the Zeiss IOL to the sclera in a sutureless manner by using the Yamane technique of intrascleral haptic fixation.
It is important to set realistic patient expectations and explain that this is a rehabilitative procedure, not a refractive one. Thus, in addition to the risks mentioned above, I would counsel the patient that he will still have some residual refractive error and would likely need glasses to achieve optimal vision after this surgery.
Liliana Werner A specific pattern of calcification in an overall round configuration localized to the anterior surface/subsurface of the central/paracentral optic area of different hydrophilic acrylic IOLs fixated within the capsular bag has been described, initially following secondary anterior segment procedures using intracameral injection of air/gas.1 Later, the same pattern of calcification of hydrophilic acrylic IOLs was found to occur following secondary posterior segment procedures (with and without vitreous tamponade). This suggested that an inflammatory or metabolic change in the aqueous humor due to repeated intraocular procedures was the cause of this secondary IOL calcification.2
Indeed, the calcification always occurs where the aqueous humor keeps contact with the anterior IOL surface within the opening area of the capsulorrhexis. If the IOL is not in the bag (e.g., fixated to the sclera), the calcified deposits may be observed in a more widespread area on the IOL’s anterior surface/subsurface. This complication has not been linked to a specific IOL design or manufacturer, but all IOLs involved are made of hydrophilic acrylic material.
Once the calcification develops, it has a significant impact on patients’ visual function. Complaints are usually related to decreased VA of at least 2 Snellen lines as well as to “hazy” vision and glare.
The patient’s symptoms may worsen with time, as the calcified deposits may start to form multilayers on the IOL surface, with a more significant degree of opacification. There is no other known treatment, and explantation/exchange is necessary to improve the associated symptoms. Retrospective studies have shown that the rate of localized calcification after posterior lamellar keratoplasty techniques could be between 5% and 9.7%.
___________________________
1 Werner L et al. J Cataract Refract Surg. 2015;41(1):199-207.
2 Balendiran V et al. J Cataract Refract Surg. 2019;45(12):1801-1807.
Q8.4 How would you manage the patient’s glare due to the iris transillumination defects?
| Wear sunglasses (i.e., continue to live with it) |
43.5%
|
| Wear a colored contact lens |
26.1%
|
|
Use miotic drops
|
17.4%
|
|
Insert an artificial iris implant
|
13%
|
|
Would have preferred Option 4, but not with a history of UGH
|
0%
|
Mitch Weikert After three years of iris chafing and UGH syndrome due to sulcus placement of a single-piece toric IOL, this patient has been left with a poorly constricting pupil and diffuse iris transillumination defects. And although his vision improved following removal of the sulcus IOL and implantation of a suture-fixated PC toric IOL, he continued to experience significant glare and “white out” of his vision. The retroillumination slit-lamp photos really demonstrate the extent of his iris atrophy.
Managing these patients can be quite challenging. The audience’s preferred choice of telling the patient to “live with it” (44%) certainly reflects this difficulty and how we may feel somewhat helpless in our ability to offer a great solution. This most popular management choice was followed by a colored contact lens (26%), miotic drops (17%), and, finally, an artificial iris implant (13%).
Colored contact lenses can help block peripheral light, but they carry several disadvantages. Patients in this age group may not tolerate contact lenses due to ocular surface disease and/or may find them difficult to insert and remove. Also, the contacts that are most effective in blocking light are not typically available in daily wear or single-use varieties. While miotic drops may reduce excess light by constricting the pupil, they’d have limited utility in cases such as this, given the extensive iris atrophy and minimal function of the pupillary sphincter.
An artificial iris implant is a viable option for this patient. While several of these devices are available worldwide, in the United States, our choice is limited to a single one, the HumanOptics Artificial Iris. This silicone prosthesis, which was approved by the FDA in May 2018, is an effective functional and/or cosmetic solution for both focal and diffuse iris defects. Each prosthesis is custom painted to match each patient’s fellow eye—or, in cases of bilateral aniridia, the patient’s preferred color choice. The implant comes in two varieties (silicone only or silicone with a mesh backing), and it can be implanted with one of three fixation techniques: 1) within the capsule, 2) passively in the sulcus, or 3) sutured in the sulcus.
This patient was tested with a colored contact lens, which successfully reduced his glare. As there was no capsule support, the iris prosthesis had to be implanted in the sulcus. Passive fixation would have had a high risk of continued UGH syndrome, so suture fixation of the mesh-backed prosthesis was the best option. The artificial iris was sutured in a location separate from the IOL sutures, so it could more easily be removed in the event of postoperative chafing and UGH syndrome. After the diameter of the patient’s ciliary sulcus was measured, the prosthesis was trephined to an appropriate size, inserted through a small incision, and fixated with Gore-Tex sutures. He experienced resolution of his glare and substantial improvement of his visual function, but he will still be monitored periodically for development of UGH syndrome.
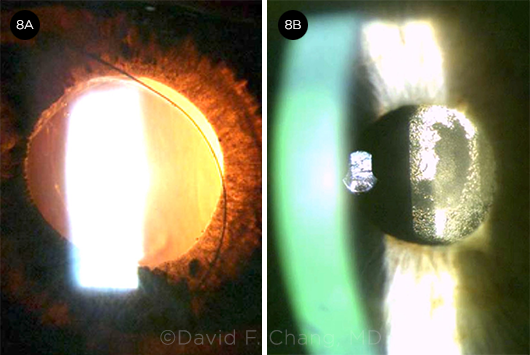 |
|
CASE 8. (8A) In this patient, symptomatic, diffuse iris transillumination defects complicated sulcus placement of a single-piece acrylic IOL. (8B) Three months following IOL exchange with a scleral Gore-Tex suture–fixated hydrophilic acrylic IOL, localized calcification of the optic reduced the BCVA to 20/200.
|
How to Access Cataract Spotlight Online
View the session. If you registered for AAO 2021, you may still view content online at aao.org/myonlineproducts. To access the Cataract Spotlight session, log in and choose AAO 2021. Virtual Meeting content will be available through Aug. 1, 2022.
View the videos. Go to aao.org/cataract-spotlight-aao2021.
|
Financial Disclosures
Amar Agarwal, MD: Jaypee-Highlights Medical Publishers: P; Slack: P; Staar Surgical: C; Thieme Medical Publishers: P. Ashvin Agarwal, MD: Elisar: O. Zaina N. Al-Mohtaseb, MD: Alcon: C; Bausch + Lomb: C; Carl Zeiss: C; CorneaGen: C. Steve A. Arshinoff, MD: Alcon: C; ArcticDx: C,O; Bausch + Lomb: C; Carl Zeiss: C; Cima Technology: C; Entod International: C; Rayner Intraocular Lenses: C. Brandon Ayres, MD: Alcon: C,L; Allergan: C,L; Bausch + Lomb: L; Carl Zeiss: C; CorneaGen: C; Dompé: C,L; Glaukos: C; New World Medical C; Novartis (Alcon Pharmaceuticals): C; Omeros: C; Sun Ophthalmics: C,L; Tear Lab: C. John P. Berdahl, MD: Aerie: C; Aerpio: C; Alcon: C; Allergan: C,L; Aurea: C,O; Avedro: C; Bausch + Lomb: C; Calhoun Vision: C; Carl Zeiss: C; Equinox: C,O; Glaukos: C,O; Iacta Pharmaceuticals: C; Imprimis: C,P; iRenix: C; Johnson & Johnson: C; Kala: C; Kedalion: C; Melt Pharmaceuticals: C; MicroOptx: C; New World Medical: C; Ocular Surgical Data: C,O; Ocular Therapeutix: C; Omega Ophthalmic: C,O; Orasis: C; Oyster Point: C,O; RxSight: C; Sight Sciences: C; Surface: C,O; Tarsus: C; Tear Clear: C; Verana Health: O; Vertex Ventures: C; ViaLase: C; Visionary Ventures: C; Vittamed: C. David F. Chang, MD: Beyeonics: C,O; Carl Zeiss: C; Centricity: C,O; Eyenovia: O; ForSight Robotics: C,O; ForSight Vision 6: C,O; Iantrek: C,O; iDrops: C,O; Ivantis: C,O; JelliSee: C,O; Johnson & Johnson: C; Long Bridge: C,O; Orasis: O; Perfect Lens: C,O; Presbyopia Therapies: O; RxSight: C,O; Slack: P; Surface: O; Versant Ventures: O; Viewpoint: C,O; Visionary Ventures II: O. Uday Devgan, MD: Advanced Euclidean Solutions: O; CataractCoach.com: O; IOLcalc.com: O; LensGen: O. Deepinder K. Dhaliwal, MD: Allergan: C; Avedro: S; Haag-Streit Group: C; Horizon: C; Johnson & Johnson: C; Novartis: C,S; Noveome: S; Ocular Therapeutix: L,C; Staar Surgical: L; Trefoil: C. Kendall E. Donaldson, MD: Alcon: C,L; Allergan: C; Avellino Labs: C; Carl Zeiss: C; Eyevance: C; Johnson & Johnson: C; Kala: C; Lensar: C; Lumenis Vision: C; Novartis: C; Omeros: C; ScienceBased Health: C; Shire: C; Sun: C; TissueTech: C. Eric D. Donnenfeld, MD: Alcon: C; Allergan: C,L; Avellino Labs: C; Bausch + Lomb: C; Beaver-Visitec International: C; BlephEx: C,O; Carl Zeiss: C; CorneaGen: C,O; Eyepoint Pharma: C; Glaukos: C; Ivantis: C; Johnson & Johnson: C; Katena Products: C,O; LacriScience: C,O; Mynosys: C,O; Novartis: C; Ocular Therapeutix: C; Omeros Corporation: C; PRN Physician Recommended Nutraceuticals: C,O; TLC Laser Center: C. Marjan Farid, MD: Allergan: C; Bausch + Lomb: C; Bio-Tissue: C; Carl Zeiss: C; CorneaGen: C; Dompé: C; Johnson & Johnson: C; Kala: C; Novartis (Alcon Pharmaceuticals): C; Sun Ophthalmics: C; Tarsus: C. Nicole R. Fram, MD: Alcon: C,L; Allergan: C; Beaver-Visitec International: L; CorneaGen: C,O,L; Dompé: C,L; Johnson & Johnson: C; MicroSurgical Technology: L; Novartis: L; Ocular Science: O; Ocular Therapeutix: C,L. Bonnie An Henderson, MD: Alcon: C,L. Richard S. Hoffman, MD: MicroSurgical Technology: C. Sumitra S. Khandelwal, MD: Alcon: C; Bausch + Lomb: C; Carl Zeiss: C; Dompé: C; Kala: C; Ocular Therapeutix: C; Omeros: C. Douglas D. Koch, MD: Alcon: C; Bausch + Lomb: C; CapsuLaser: O; Carl Zeiss: C; Ivantis: O; Johnson & Johnson: C; Perfect Lens: C; Vivior: O. Thomas Kohnen, MD, PhD, FEBO: Allergan Medical Affairs: C; Bausch + Lomb: C; Carl Zeiss: C,S; Dompé: C; Geuder: C; Hoya: S; Johnson & Johnson: C,S; MedUpdate: C; Merck: C; Novartis (Alcon Pharmaceuticals): C,S; Oculentis: C,S; Oculus Optikgeräte: C,S; Presbia: C,S; Rayner Intraocular Lenses: C; Santen: C; Schwind eye-tech-solutions: C,S; Staar Surgical: C; TearLab: C; Thea Pharma: C; Thieme Compliance: C; Ziemer: C. Richard L. Lindstrom, MD: AcuFocus: C,O,P; Alcon: C; Allegro: C,O; Alphaeon: C,O; Aprea Therapeutics: O; Arixa: C,O; Augustine Temperature Management: O; Avellino Labs: C,O; Aviana: O; Bausch + Lomb: C,O,P; Belkin Laser: C,O; Broadspot: C,O; Bruder: O; Carl Zeiss: C; Centricity Vision: C,O; Checked-Up: C,O; Combangio: C,O; Confluence Acquisition Partners: O; CorneaGen: C,O; CXL, ESI: C,O; Digital Systems: O; Dose: C,O; E-vision Medical Devices: O; E-vision: O; EBV Partners: O; Egg Basket Ventures: O; Egg Factory: O; Equinox: C,O; Eton: O; Evolus: O; Excel-Lens: O; Expert Opinion MD: C,O; Eye Safe: C,O; Eyemaginations: C,O; Flying L Ventures: C,O; ForSight 4: O; ForSight Vision 3 - Drug Delivery Posterior Segment: O; ForSight Vision 6 - Accommodating IOL: C,O; Freedom Software: O; FZioMed: O; Glaukos: C,O; Gondola: C,O; Harrow Health: C,O,P; Health-E: C,O; Healthcare Transaction Services: C,O; High Performance Optics: O; Iantrek: O; IDoc: O; Imprimis: C,O; Injectsense: C,O; Innovega: O; iOR: C,O; Johnson & Johnson: C; KalaRx Pharmaceuticals: C,O; Kedalion: C,O; KeraFlow: C,O; Lensar: C,O; LenTechs: C,O; Lenticular Research Group: O; Lifeguard Health: C,O; Lumineyes: O; MD Backline: C,O; Melt: C,O; Minnesota Eye Consultants: C,O; NASA-Vision for Mars Program: C; Neurovision: C,O; New Sight Reality: O; Novabay: C,O; Nutraceutical Holdings: C,O; Ocular Optics: C,O; Ocular Surgery News: C; Ocular Therapeutix: C,O; Oculatec: O; Ocutrx: C,O; Omega Eye Health: C,O; Omega Ophthalmics: C,O; Omeros Corp: C; Orasis: C,O; Oyster Point: C,O; PogoTec: C,O; Q Sensei: O; Quest: C,O,P; Revana: O; RxSight: C,O; Schroeder Ventures Fund II, III, IV, VI: C,O; SightPath: C,O; Silk Technologies: C; Solbeam: O; Strathsprey Crown: O; Stroma: O; SunPharma: C; Surface Pharmaceuticals: C,O; Tarsus: C,O; TearClear: C; TearLabs: C,O; TherOptix: C,O; TissueTech: O; Tracey Technologies: C,O; True Iris: C,O; True Vision: C,O; Versant Fund Side Fund: C,O; Vinci: C,O; Viradax: O; Vision Solutions Technology: C,O; Visionary Ventures: C,O. Nick Mamalis, MD: Advanced Vision Science: S; Alcon: S; Anew Optics: C,S; Atia Vision: S; Carl Zeiss: S; ClarVista: S; Clearsight: S; CoDa Therapeutics: S; Cord: S; Genisphere: S; Hoya: S; KeraMed: S; LensGen: S; Medicontur: S; Merck: S; Mynosys: S; Omega: S; PerfectLens: C,S; Physiol: S; PowerVision: S; Qura: S. Yuri McKee, MD: Carl Zeiss: C; Interactive Medical Publishing: O; Ivantis: C. Cathleen M. McCabe, MD: AbbVie: C; Alcon: C,L,S; Allergan: C,S; Bausch + Lomb: C,L; Carl Zeiss: C,L; Dompé: C; EyePoint: C,L,S; Glaukos: L,S; Ivantis: C,L,O,S; Johnson & Johnson: S; Novartis: C,L; Ocular Innovations: C,O; Ocular Therapeutix: C,L,S; Omeros: C,L; Ora: S; ScienceBased Health: C; Sight Sciences: C; Sun Ophthalmics: C,L; Tarsus: C. Kevin M. Miller, MD: Alcon: C; Johnson & Johnson: C,S; Lensar: C; Oculus: C. Rudy Nuijts, MD: Alcon: C,L,S; Carl Zeiss: S; Johnson & Johnson: S; Ophtec: L. Thomas A. Oetting, MD: None. Amandeep S. Rai, MD: Alcon: C,S; Bausch + Lomb: C,S. Naveen K. Rao, MD: Parexel: C; W.L. Gore: C. Steven G. Safran, MD: Haag-Streit Group: L. Thomas W. Samuelson, MD: Aerie Pharmaceuticals: C; Alcon Surgical: C; Allergan: C; Bausch + Lomb/Valeant: C; Belkin Laser: C,O; Carl Zeiss: C; ELT Sight: C; Equinox: C,O; Glaukos: C; Imprimis: C; Ivantis: C,O; Johnson & Johnson: C; MicroOptx: C; New World Medical: C; Ocuphire: C,O; PolyActiva: C; Ripple Therapeutics: C; Santen: C; Sight Sciences: C,O; TearClear: C,O; Vialase: C,O. Julie M. Schallhorn, MD: Carl Zeiss: C; Long Bridge: O; Vanda: C. I. Paul Singh, MD: Allergan: L; Bausch + Lomb: C,L; ellex: C,L; Glaukos: C; iStar Medical: L; Ivantis: C,L; Katena: C; New World Medical: C,L; Novartis (Alcon Pharmaceuticals); Omeros: L. Michael E. Snyder, MD: Alcon: S; Alnivo: C; Glaukos: S; Haag-Streit USA: C; HumanOptics: C,P; Johnson & Johnson: S; Trefoil: S; Veo Ophthalmics: O; W. L. Gore: C. Vance M. Thompson, MD: AcuFocus: C,L,O; AI Optics: O; Alcon: C,L; Allergan: C; Allotex: C,O; Avellino Labs: C,O; Avisi Technologies: C,O; Bausch + Lomb: C; Brim Biotechnology: C; Costruzioni Strumenti Oftalmici: C; Carl Zeiss: C; Centricity Vision: C,L,O; Conjtac: C,O; D & D Biopharmaceuticals: C,O; Equinox: C,O; Euclid Systems: C,L,O; Expert Opinion: C,O; Eyebrain Medical: C,O; Eyedetec: C,O; EyeGate Pharma: C,O; FemtoVision: C,O; ForSight Robotics: C,O; Glaukos: C,L; Healthe: C,L,O; Imprimis: C; iVeena: C,O; Johnson & Johnson: C,L; Leica Microsystems: C; Melt Pharmaceuticals: C,O; Ocular Innovations: C,O; OneFocus: C,O; ORA: C; Oyster Point Pharma: C,O; Percept: C,O; Refocus Group: C; RxSight: C,O; Sight Sciences: C,O; Stuart Therapeutics: C,O; Tarsus Rx: C,O; TearClear: C,O; TherOptix: C,O; Treehouse Health: C,O; Veracity: C,O; Visant: C,O; Visus: C; Vivior AG: C,O. John A. Vukich, MD: AcuFocus: C; Johnson & Johnson: C; Staar Surgical: C. Keith A. Warren, MD: Allergan: C. Mitchell P. Weikert, MD: Alcon: C; Carl Zeiss: C. Liliana Werner, MD, PhD: Alcon: C,S; Bausch + Lomb: C,S; Carl Zeiss: S; Cristalens Industrie: S; Hoya: C,S; LensGen: S; Long Bridge Medical: S; Morcher: S; OnPoint Vision: S; PerfectLens: S; Physiol: S; Shifamed/Atia: S; Spyglass: S. William F. Wiley, MD: Alcon: C,L; Allergan: C; ArcScan: C; Carl Zeiss: C; CorneaGen: C; Equinox: C,O; Glaukos: C; Imprimis: C,P; Ivantis: C; Johnson & Johnson: C; New World Medical: C; Omega Lens: C,O; Revision Optics: C,L. Elizabeth Yeu, MD: Alcon: C,L; Allergan: C,L; ArcScan: C; Bausch + Lomb: C; Bio-Tissue: C,L; Carl Zeiss: C,L; Glaukos: C,L; Innovation Labs: O; iOptics: C,S; Johnson & Johnson: C,L; Kala: C,S; Katena Products: C; Melt: C,O; Modernizing Medicine: O; Novartis (Alcon Pharmaceuticals): C; Ocular Science: C,O; OcuSoft: C; Omeros: L; ScienceBased Health: C; Sight Sciences: C; Sun Ophthalmics: L; Surface: C; Tarsus: C,O; TearLab: C; TearScience: C; TissueTech: C,L; Topcon Medical Systems: S.
See the disclosure key at www.aao.org/eyenet/disclosures.
|