By Bea Palileo, MD, Nicolas Kang, BS, and Frank S. Hwang, MD
Edited By: Bennie H. Jeng, MD
Download PDF
Radial keratotomy (RK) is a type of keratorefractive surgery that was commonly performed in the 1980s and early 1990s. As the population ages, an increasing number of post-RK patients are now requiring cataract surgery. The complications that occur in these patients can be divided into two major categories: difficulty in achieving the post–cataract surgery target refraction and decreased corneal integrity from compromised RK incisions.
Long-term sequelae of the oblate-shaped, post-RK cornea—such as progressive hyperopic shift, diurnal variations in refraction, and increase in corneal astigmatism—require extensive planning before cataract surgery to achieve the refractive target. In addition, preventing intraoperative and postoperative complications is critical in maintaining corneal integrity and optimizing refractive outcomes.
Preoperative Considerations
In a post-RK cornea, IOL power predictions are affected by refractive changes induced by both cataract progression and corneal surface irregularities. Thus, these eyes warrant special consideration.
Preoperative evaluation. Corneal tomography or scanning-slit interferometry is preferred over corneal topography in patients with a history of RK. Tomography can evaluate both anterior and posterior corneal surfaces and provide more accurate assessment of corneal power and astigmatism.1
Traditionally, an evaluation of total corneal power was performed with the use of a rigid gas-permeable contact lens to compensate for corneal surface irregularities and thus reduce errors in IOL power prediction.1 However, this type of evaluation is rarely used now, as web-based tools such as the American Society of Cataract and Refractive Surgery (ASCRS) postrefractive IOL calculator provide better utility and ease of use.
IOL power calculation. IOL calculations after RK are generally more problematic than those after laser corneal ablation because of refractive instability and irregular astigmatism. Unlike laser-ablated corneas, post-RK corneas have a relatively proportional flattening of both anterior and posterior corneal surfaces.1 Thus, in the prediction of corneal power, a constant relationship between the anterior and posterior curvature of the cornea is maintained. Additionally, in post-RK patients, the corneal curvature may progressively change over time, inducing a hyperopic shift. Many of these patients also have extremely flat corneas, resulting in poor quality of vision due to higher- and lower-order aberrations.
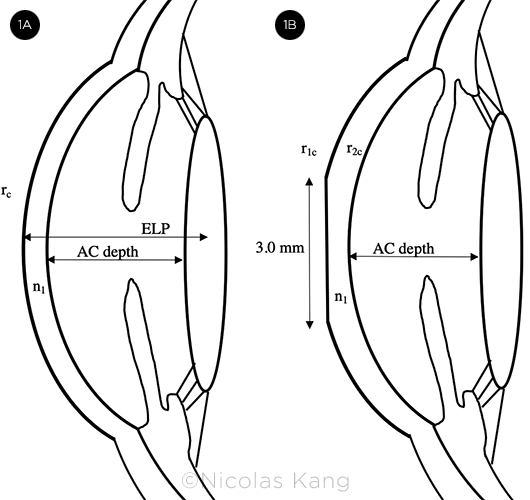
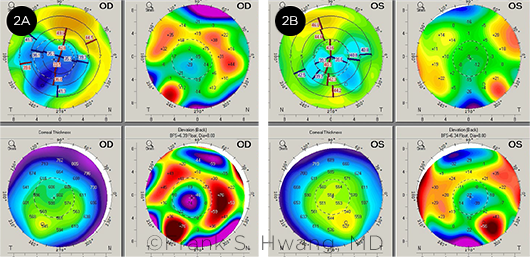
Other changes are worth noting. First, unlike pre-RK corneas (Fig. 1A), post-RK corneas have a 3-mm zone of central flattening (Fig. 1B), which can be problematic because conventional topography and keratometry measure up to 4 mm of the paracentral zone.2 Measurements in the 4-mm paracentral zone of these eyes overestimate corneal power and lead to postoperative hyperopia.2 Second, surface irregularities after RK result in various steep and flat areas that do not align (Fig. 2), making the determination of the net steep and flat meridians difficult.1 Finally, intraoperative manipulation of the cornea and postoperative corneal instability further add to the challenges in achieving refractive targets with cataract surgery.
Refractive target. Because of the corneal changes described above, many authors suggest aiming for a myopic correction between –0.50 D and –1.50 D to avoid postoperative hyperopic surprises.2,3 This target addresses concerns about overestimating the true corneal refractive power and the progressive hyperopic shift up to 10 years after RK. Chen et al. showed that implantation of an IOL aiming for plano in 24 post- RK eyes would have resulted in hyperopic refraction in 83.4% of cases and that the hyperopic shift after cataract surgery could be reduced (though not eliminated) if the surgeon used a myopic target of –1.50 D.2
IOL power calculation formula. Turnbull et al. recommended the following IOL calculation formulas, depending on the availability of the patient’s refractive history.4
When refractive history is available:
- Barrett True K [History]
- Barrett True K [Partial History]
When refractive data before and after RK are not available:
- Barrett True K [No History]
- Standard Haigis formula
The True K and standard Haigis methods performed significantly better than the others in this study: DK-Holladay-IOLM, Potvin-Hill, or Haigis (with –0.5-D offsets).4
The updated RK algorithm in True K (version 2.0, 2016) applies theoretical models of refractive changes to calculate pre-RK keratometry and predict the posterior corneal contribution. Results are then uploaded to the ASCRS online IOL calculator (https://iolcalc.ascrs.org/). The updated True K does not use population means for calculations and can be more accurate than formulas using standard K methods.
The advantage of True K [History] is that the magnitude of RK correction is known. However, it may be difficult for the surgeon to obtain this data. True K [Partial History] uses the post-RK refraction and current biometry to estimate preoperative corneal curvature and effective lens placement. The True K [No History] formula predicts changes in refraction using the axial length, anterior chamber depth, and current keratometry to estimate the preoperative keratometry.4 (See Table 1 for a comparison of the formulas’ accuracy.)
 |
|
CENTRAL FLATTENING. (1A) Pre-RK anterior segment with effective lens placement (ELP), central radius of corneal curvature (rc), and keratometric index of refraction (n1). (1B) Post-RK anterior segment with a 3.0-mm flat central cornea and proportionally flattened anterior (r1c) and posterior (r2c) corneal surfaces 3 to 4 mm paracentrally.
|
Intraocular Lens Selection
Higher-order aberrations. Anterior corneal spherical aberration should be measured at a 6-mm aperture before cataract surgery. Post-RK corneas have abnormal curvatures that result in higher-order aberrations. Proper IOL selection can minimize this effect; the use of negative spherical aberration aspheric IOLs may be preferable for oblate-shaped corneas after RK.1
Controversy. The use of multifocal IOLs and small-aperture IOLs in post-RK patients remains controversial; only small studies have been performed, including the following:
- In a case series, Agarwal et al. reported the outcome of unilateral small-aperture IC-8 IOL (AcuFocus) implantation in two patients with history of myopic RK. Both patients achieved spectacle independence while maintaining stereopsis and contrast sensitivity.5
- Gupta et al. studied two patients who attained spectacle independence after implantation of an AcrySof IQ ReSTOR multifocal IOL (Alcon) in the nondominant eye and a Tecnis ZCB00 one-piece acrylic IOL (Abbott Medical Optics) in the dominant eye.6
- Nuzzi et al. reported a case in which a multifocal toric IOL offered acceptable visual acuity without requiring correction.7
Patient education. Surgeons should discuss with patients the challenges of obtaining emmetropia after cataract surgery, refractive errors from IOL model choice, and the possible need for distance glasses with a myopic refractive target.1
 |
|
IRREGULAR ASTIGMATISM. Corneal tomography showing irregular astigmatism in both the (2A) right and (2B) left eyes.
|
Intraoperative Considerations
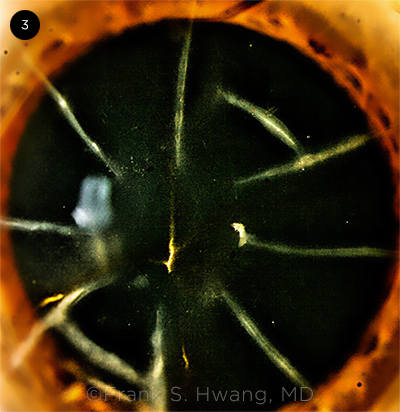
Phacoemulsification incisions. The surgeon should take care to avoid intersection of the main entry incision with the RK incisions (Fig. 3).8
- To reduce the risk of dehiscence, clear corneal incisions can be placed peripherally between two adjacent RK incisions, using a size of 3.2 mm or less with eight RK incisions; 2.2 mm or less with 12 RK incisions; and 2.0 mm or less with 16 RK incisions.9
- If the incision is to be placed in the cornea, a temporal approach is recommended, and a precautionary stabilizing suture may help prevent intraoperative dehiscence. Meduri et al. studied 24 patients with 16 RK incisions and found that 37.5% of cases with wound dehiscence had superior incisions without sutures, 20% had temporal incisions without sutures, and 0% had temporal incisions with a stabilizing suture.10
If the number of radial incisions does not allow for adequate placement of a clear corneal incision for phacoemulsification, a scleral tunnel incision is the procedure of choice.8,9
Intraocular pressure (IOP). Lowering IOP decreases the risk of RK wound dehiscence. Keep IOP low by taking care not to overfill the anterior chamber with viscoelastic, avoiding high pressures during hydrodissection, and monitoring bottle height.9
Wound dehiscence. Recent publications by Wang et al. and Zhang et al. suggest use of an intracameral air bubble as a sutureless approach to manage RK incision dehiscence, with or without viscoelastic agent injection.8,9 Compared with corneal sutures, advantages of this method include a shorter operative time, as well as avoidance of postoperative patient discomfort and potential corneal astigmatism. However, sutures may be necessary to stabilize the corneal wound in eyes with persistent leakage and larger ruptures. Meduri et al. showed that if dehiscence of an RK incision occurs, a 10.0 nylon suture can be placed at the time of surgery and removed two weeks later.10
 |
|
RK SCARS. Slit-lamp photograph showing eight RK incisions and multiple astigmatic keratotomy incisions.
|
Postoperative Considerations and Follow-up
Hyperopic shift. A minimum follow-up period of three months is recommended because of transient hyperopic shifts after cataract surgery.4 The shift is caused by swelling of the RK incision, which widens the incision and induces temporary corneal flattening. Thus, initial postoperative hyperopia is normal and should not prompt a premature IOL exchange or new prescription for glasses. The final refractive outcome of an RK eye typically manifests six to 12 weeks after cataract surgery, after full healing and stabilization of the corneal curvature.1
Corneal changes. After surgery, there may be decreased endothelial density, which is associated with high myopia, impaired corneal integrity from RK, and increased intraoperative time during cataract extraction.9 Furthermore, postoperative aberrations may be present as a result of corneal irregularities. Management often includes scleral or rigid gas-permeable lenses.1
Refractive surprise. Several options are available to improve postoperative refractive errors and astigmatism.1 These include spectacles or rigid gas-permeable contact lenses, and lens-based procedures (IOL exchange or piggyback IOL implantation).
Limbal relaxing incisions are not commonly used after RK because of poor predictability and possible exacerbation of ocular surface disease.
Table 1: Comparison of IOL Formula
| Formula |
MAE |
MedAE |
%
±0.50 D |
%
±1.00 D |
| True K [History] |
0.369 |
0.275 |
76.6 |
97.9 |
| True K [Partial History] |
0.374 |
0.382 |
75.0 |
95.8 |
| True K [No History] |
0.418 |
0.330 |
69.2 |
92.3 |
| Haigis |
0.425 |
0.406 |
69.2 |
92.3 |
|
MAE = mean absolute error; MedAE = median absolute error.
SOURCE: Adapted from Turnbull AMJ et al. Ophthalmology. 2020;127(1):45-51, Table 2.
|
Conclusion
Patients with prior RK who undergo cataract surgery may experience two significant complications: difficulty in achieving the post–cataract surgery target refraction and decreased corneal integrity. Evaluating these patients by means of corneal tomography and aiming for a myopic refraction between –0.50 D and –1.50 D may minimize postoperative hyperopia. Turnbull et al.4 suggest that the Barrett True K [History] or True K [Partial History] formulas are the preferred choices to achieve the refractive target. If no refractive history is available, the True K [No History] and standard Haigis formulas provide good alternatives. Intraoperatively, it is of paramount importance to decrease the possibility of RK wound dehiscence by avoiding the intersection of the main entry incision with the prior RK incisions and by preventing high IOP. These measures reduce the likelihood of refractive surprise following cataract surgery in post-RK eyes.
__________________________
1 Alio JL et al. Surv Ophthalmol. 2016;61(6):769-777.
2 Chen L et al. J Cataract Refract Surg. 2003;29(1):65-70.
3 Geggel HS. Ophthalmology. 2015;122(5):897-902.
4 Turnbull AMJ et al. Ophthalmology. 2020;127(1):45-51.
5 Agarwal S, Thornell EM. J Cataract Refract Surg. 2018;44(9):1150-1154.
6 Gupta I et al. Case Rep Ophthalmol. 2014;5(2):157-161.
7 Nuzzi R et al. Case Rep Ophthalmol. 2017;8(2):440-445.
8 Wang JD et al. J Int Med Res. 2020;48(3):300060519895679.
9 Zhang JS. PLoS One. 2016;11(12):e0165474.
10 Meduri A et al. Int J Ophthalmol. 2017;10(7):1168-1170.
__________________________
Dr. Palileo is an ophthalmology resident at Loma Linda University Eye Institute in Loma Linda, California; Mr. Kang is a fourth-year medical student at Loma Linda University School of Medicine; and Dr. Hwang is a cornea specialist at Loma Linda University Medical Center and assistant professor at Loma Linda University Eye Institute. Financial disclosures: None.