Download PDF
As more of the targeted cancer drugs known as immune checkpoint inhibitors (ICIs) are introduced—and as the indications for their use expand—ophthalmologists are being challenged to keep up with their potential ocular adverse effects.
While the literature supports a multidisciplinary approach to dealing with such immune-related adverse events (AEs), it has offered no roadmap. Now, researchers at Johns Hopkins University in Baltimore have shown a way. They report a multidisciplinary collaboration in which oncologists, ophthalmologists, and other specialists successfully diagnosed and managed patients with immune-related AEs.1 The team approach proved both feasible and useful, the researchers said.
“We are the first group to show that oncology providers deferred to team recommendations on immune-related AE diagnosis and management,” said oncologist and lead author Jarushka Naidoo, MBBCh.
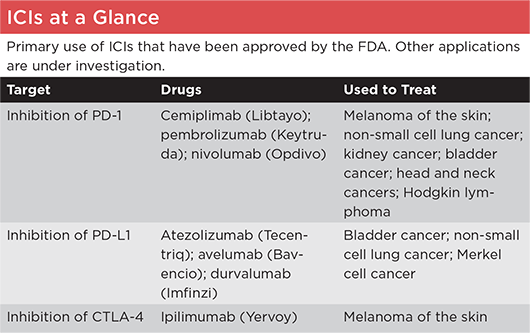 |
PD-1 = programmed cell death protein 1; PD-L = programmed death-ligand 1; CTLA-4 = cytotoxic T-lymphocyte–associated protein 4.
SOURCE: American Cancer Society
|
Multispecialty investigation. The pilot collaboration involved eight oncologists, four oncology nurses, and an immune-related toxicity team of 21 medical specialists, including two ophthalmologists. They interacted through an electronic messaging forum where oncologists could seek diagnostic and management assistance from the medical specialists, who were to respond via e-mail within 24 hours. In all, 117 oncology consultations (involving 102 patients treated with ICIs) were made over eight months ending in March 2018.
Ocular and other adverse events. Pneumonitis topped the list of confirmed toxicities, followed by arthritis, dermatitis, and diarrhea/colitis. In the pilot program, the only ocular event, a case of bilateral anterior uveitis, resolved with topical steroids, per the advice of team ophthalmologist Meghan Berkenstock, MD.
Other researchers have found a broader range of ocular immune-related AEs, including corneal perforation, corneal punctate epithelial erosion, subconjunctival hemorrhage, uveitis, hypotony maculopathy, cystoid macular edema, retinal detachment, choroiditis, optic neuritis, melanoma-associated retinopathy, and visual field loss related to antiretinal and anti-optic-nerve autoantibodies.2
A growing concern. The current evidence indicates that ophthalmic complications occur in approximately 1% of patients on ICIs.2 But as Dr. Berkenstock pointed out, “These side effects will be more common in the future, given the expanding indications for use of these drugs.” (The checkpoint inhibitors are licensed for at least 13 cancer types.)
Thus, ophthalmologists need to watch for new symptoms in patients who have been treated with an ICI—even if the infusion took place several weeks prior to their ophthalmologic appointment.
And close collaboration with the patient’s oncologist and other specialists should be part of the ophthalmologist’s treatment plan, Dr. Berkenstock cautioned. “Hence, the need for a toxicity team to facilitate this communication.”
—Miriam Karmel
___________________________
1 Naidoo J et al. J Natl Compr Netw. 2019;17(6):712-720.
2 Kim JM et al. Ophthalmology. 2019;126(7):1058-1062.
___________________________
Relevant financial disclosures—Dr. Berkenstock: None. Dr. Naidoo: AstraZeneca/MedImmune: C,L,S; Bristol-Myers Squibb: C,L; Calithera: S; Kyowa Hakko Kirin: S; Merck: S; Takeda: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Berkenstock None.
Dr. Chew None.
Dr. Masket Accutome: S; Alcon: C,L; CapsuLaser: C,O; Haag-Streit: C,P; Morcher: P; Ocular Science: C,O; Ocular Theraputix: C,O; PowerVision: C; VisionCare Ophthalmic Technologies: C.
Dr. Naidoo AstraZeneca/MedImmune: C,L,S; Bristol-Myers Squibb: C,L; Calithera: S; Kyowa Hakko Kirin: S; Merck: S; Takeda: C.
Dr. Rowan None.
Dr. Taylor None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review