Download PDF
In keeping with the rest of AAO 2020 Virtual, the 19th annual Spotlight on Cataract Surgery was the first fully virtual rendition. Cochaired by Nicole R. Fram, MD, and myself, the symposium was called “Complicated Phaco Cases—My Top 5 Pearls” (Spo3V and Spo4V).
In this format, each of the eight faculty members was given only 7 minutes to highlight their five best pearls for a specific category of challenging problems. This year’s topics included phaco with uveitis (Jessica Ciralsky) or with glaucoma (Douglas Rhee), preventing and managing CME (Rudy Nuijts), reducing perioperative drops (Neal Shorstein), toric IOL planning and alignment (Warren Hill), using the light-adjustable IOL (John Berdahl), patient selection and education for advanced technology IOLs (Richard Tipperman), and knowing when and how to exchange a refractive IOL (Zaina Al-Mohtaseb). After each presentation, a panel of experts added their own pearls and comments. Audience questions were posed and answered online in real time.
An additional five lectures were prerecorded and presented in the on-demand section of the meeting platform. These topics were phaco with small pupils (Rosa Braga-Mele, Spo3.01), posterior polar cataract (Abhay Vasavada, Spo3.04), pars plana anterior vitrectomy (Kevin Miller, Spo3.02), pseudophakic dysphotopsia (Sam Masket, Spo3.03), and eyes with high hyperopia and a crowded anterior chamber (Doug Koch, Spo3.05).
For this article, we’ve asked our eight faculty members to review their top five pearls. We look forward to returning live to the 2021 annual meeting, when the Spotlight on Cataract Surgery will celebrate its 20th anniversary.
—David F. Chang, MD
Cataract Spotlight Program Cochairman
SCENARIO 1
Phaco in Patients With Uveitis
JESSICA B. CIRALSKY, MD
1. Identify the underlying etiology. You should always try to identify the underlying cause of uveitis. You especially need to rule out any infectious cause because you are going to treat these cases very differently [from noninfectious ones]. It is also important to know the underlying cause, as different diseases act differently with cataract surgery. For example, patients with juvenile idiopathic arthritis typically have severe inflammation postoperatively whereas those with Fuchs heterochromic iridocyclitis have a mild course. Knowing which disease you are treating will help guide your therapy.
Control of the underlying problem is important regardless of the cause. I like to have at least three months of complete inflammatory control before operating. Complete inflammatory control means no cells in the anterior chamber and minimal vitreous inflammation.
2. Plan ahead for surgery. Think about the patient’s other ocular pathology before getting to the operating room (OR). If a patient has corneal pathology that will impede the view, you should treat this ahead of time. If there is concomitant glaucoma or retinal pathology, consider combined or sequential surgeries. You should also have a plan and a backup plan for the OR. I often block these patients to ensure that there is adequate anesthesia because these are typically complex surgeries. I also like to have a contingency tray ready with everything I may need in the surgery to deal with all of the possible complications that may arise.
3. Control the iris. Synechiae, pupillary membranes, and miosis are common in uveitic cases. For posterior synechiolysis, I start under a nonadhered area and then sweep with a viscoelastic cannula. For adhesions and pupillary membranes, I often use retinal microscissors to remove them as these are often thick and fibrotic. For iris retraction, I use iris hooks if the anterior chamber is shallow and the pupil is small. I will use a pupil expansion ring if the anterior chamber is deep and the iris is not atrophic. I do not recommend stretching an atrophic iris.
4. Choose your IOL (or lack of) wisely. Make sure that you have done meticulous surgery before inserting the IOL. All lens material should be removed, and cortical cleanup should be thorough. As for the type of IOL, I often choose a three-piece IOL in the bag. I avoid multifocal and silicone lenses. For children and those with uncontrolled chronic uveitis, I will consider leaving the patient aphakic.
5. Actively manage corticosteroids. Corticosteroids should be considered pre-, peri-, and postoperatively. I like to give oral corticosteroids (typically 1 mg/kg) starting three days before surgery, although the dose is dependent on the severity of the uveitis and the other comorbidities. I then taper it over the next month dependent on disease severity and the patient’s response. For topical medications, I use aggressive topical steroids tapered over the first few months, a topical nonsteroidal anti-inflammatory drug (NSAID) for a few months, and a topical mydriatic for a few weeks.
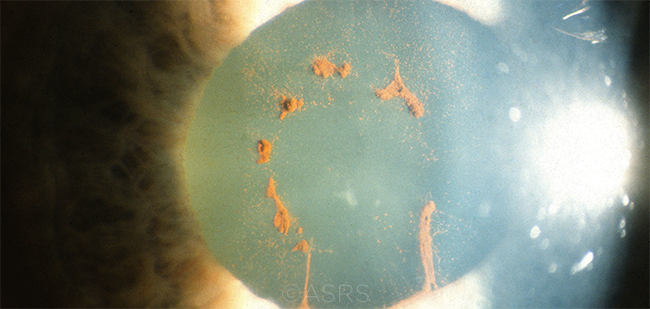 |
|
SCENARIO 1. Synechiae (shown here) are common in uveitic cases and can be swept with a viscoelastic cannula. This image was originally published in the ASRS Retina Image Bank. Jeffrey G. Gross, MD. Broken Posterior Synechia in Eye. Retina Image Bank. 2012; Image Number 1265. © The American Society of Retina Specialists.
|
SCENARIO 2
Preventing and Managing CME/CSME
RUDY M.M.A. NUIJTS, MD, PhD
1. Dual drug regimen. Patients treated with a combination of topical bromfenac 0.09% and dexamethasone 0.1% have been found to have a lower risk for developing clinically significant macular edema (CSME) after cataract surgery than patients treated with either drug singly. In the PREMED 1 study, the incidence of CSME was 1.5% for the combination regimen, versus 3.6% and 5.1%, respectively, for bromfenac and dexamethasone alone.1
2. Triamcinolone risks and benefits in patients with diabetes. In diabetic patients, a 40-mg subconjunctival injection of triamcinolone effectively prevents the development of CSME—specifically, in PREMED 2, the incidence of cystoid macular edema (CME) was 0% in those who received triamcinolone, versus 4.3% in those who did not. However, IOP elevation >25 mmHg occurred in 7.3% of cases, and this adverse effect has to be weighed in the individual diabetic patient against the risk of developing macular edema.2
3. Routine cataract surgery for those with diabetes but no DR. In diabetic patients without diabetic retinopathy (DR), we perform routine cataract surgery, using a combination of topical NSAIDs and steroids. We feel this is acceptable for the following reasons:
- In PREMED 1 and 2, the incidence of CSME was similar in the cohorts who received combination therapy. (Of note, in PREMED 2, 85% of the patients with diabetes did not have DR. In this cohort, the incidence of CSME was similar to that observed in the routine patients in PREMED 1.)
- There is a lack of evidence for the optimal dose of subconjunctival triamcinolone to prevent CSME.
- Use of triamcinolone may result in an increased risk of IOP elevation.
4. Combination of anti-VEGF drugs and steroids. Intravitreal anti-VEGF drugs and steroids can be combined with cataract surgery and are effective in preventing a postoperative increase of preexisting diabetic macular edema. A meta-analysis of 238 eyes found that macular thickness was significantly thinner and visual acuity was significantly better in those treated with intravitreal bevacizumab.
5. Postoperative management of CME. Managing CME following cataract surgery initially consists of topical NSAIDs, which can be combined with steroids; the combination regimen has been shown in small clinical studies to be more effective than NSAIDs alone. Treatment with acetazolamide has not been shown to be effective in the treatment of CME after cataract surgery.
___________________________
1 Wielders LHP et al., for the ESCRS PREMED Study Group. J Cataract Refract Surg. 2018;44(4):429-439.
2 Wielders LHP et al., for the ESCRS PREMED Study Group. J CataractRefract Surg. 2018;44(7):836-847.
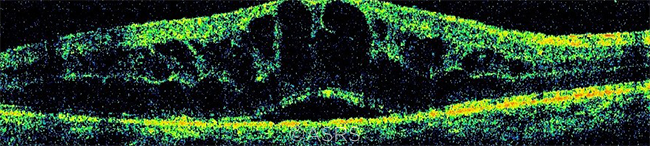 |
|
SCENARIO 2. When CME (shown here) occurs postoperatively, treatment may consist of topical NSAIDs plus steroids. This image was originally published in the ASRS Retina Image Bank. Neha Goel, MS, DNB, FRCS (Glasg). CME-OCT. Retina Image Bank. 2015; Image Number 24974. © The American Society of Retina Specialists.
|
SCENARIO 3
Dropfree Cataract Surgery
NEAL H. SHORSTEIN, MD
1. Use lidophen. Lidophen (lidocaine 1% + phenylephrine 1.5%) can be compounded by a 503b registered pharmacy and is injected at the start of surgery. The drug helps ensure maximal possible dilation when paired with cyclopentolate (instilled in the pre-op holding area, preferably 60-90 minutes prior to surgery).
2. Inject an intracameral antibiotic. We currently inject 0.5 mL of moxifloxacin 0.1% at the conclusion of surgery. (This is roughly equivalent to 0.1 mL moxifloxacin 0.5%.) One study found that when the antibiotic is used for stromal hydration, the drug persists at the wound for up to 24 hours. In the United States, moxifloxacin or cefuroxime must be compounded; 503b registered pharmacies are likely the most reliable source at this time.
Perioperative antibiotics are unnecessary. A large meta-analysis and a number of large retrospective studies have shown no benefit of topical antibiotics in preventing endophthalmitis, particularly when intracameral injection is used.
3. Inject subconjunctival triamcinolone for inflammation control. We currently inject 0.4 mL of a commercially available dilute form of triamcinolone acetonide (10 mg/mL) at the end of surgery 5-6 mm inferior to the inferior limbus. The injection balloons the conjunctiva and spreads out the steroid depot. The fluid from injection is absorbed in one to two days, while the white depot is usually visible for one to two months.
Perioperative steroids or NSAIDs are unnecessary. A number of studies show equal or better effectiveness with injected steroid. In a large prospective European study, no eyes in patients with diabetes injected with triamcinolone developed CME, although the group had a higher mean intraocular pressure (IOP), likely due to the dosage (40 mg).
4. Strategies for avoiding IOP rise and subconjunctival hemorrhage. Injecting the concentrated form of drug (40 mg/mL) or injecting either the concentrated or the dilute preparation of the drug too close to the limbus may lead to elevated IOP. Injection should be avoided in patients with advanced glaucoma and in patients younger than 65 with very long axial lengths due to higher likelihood of steroid IOP response. Acute subconjunctival hemorrhages can occur during steroid injection and can be avoided by visualizing the needle tip. Late-onset hemorrhages can occur in <1% of patients; they may recur months after surgery and have been self-limited.
5. Other preventive strategies to consider when going dropfree. Other important measures to decrease infection risk include instillation of povidone-iodine 5% solution for three to five minutes prior to surgery; careful wound construction and maintenance; leaving a relatively firm eye at the conclusion of surgery (since the IOP may drop in the hours after surgery); and stromal hydration, preferably with an intracameral antibiotic solution.
FIRST PANEL. During the first Cataract Spotlight session (Spo3V), Dr. Chang (top left) led (clockwise, from top center) Cathleen M. McCabe, MD, Eric D. Donnenfeld, MD, Marjan Farid, MD, and John A. Hovanesian, MD, in discussion.
SCENARIO 4
Phaco in Patients With Glaucoma
DOUGLAS J. RHEE, MD
1. Assess the need for a minimally invasive glaucoma surgery (MIGS) procedure. I consider adding MIGS if:
- the patient’s IOP is uncontrolled,
- the patient’s IOP is controlled but requires three or more medications,
- the patient is at high risk of experiencing a postoperative IOP spike (e.g., pseudoexfoliation syndrome), or
- there is a high risk of loss of central fixation from post-op IOP fluctuations (e.g., if one or more of the four central points of the visual field are affected).
2. Use iris hooks or rings if you suspect you will need them. I have rarely ever regretted using iris hooks or a Malyugin ring. In contrast, I have almost always regretted not using iris hooks or a Malyugin ring when I thought they would be helpful.
3. Adjust IOL calculations if needed. This is particularly necessary if a significant IOP reduction (e.g., >15 mm Hg) is anticipated. Low IOPs and hypotony cause a hyperopic shift by shortening the eye.
4. Avoid multifocal IOLs. Multifocal IOLs degrade contrast sensitivity, and glaucoma itself degrades contrast sensitivity. I would avoid these IOLs even in early stage glaucoma patients, as you cannot be assured that the patient will not progress.
5. Use topical NSAIDs if the patient is on a prostaglandin analogue (PGA). Although topical PGA use is associated with an increased risk of postoperative CME, I do not stop the use of a PGA preoperatively, as the loss of IOP control could cause permanent optic nerve damage.
Postoperatively, I do not routinely restart PGAs until I have the chance to see the IOP following surgery, because it is possible that the cataract surgery (or the cataract surgery plus a MIGS procedure) could result in a controlled IOP. Topical NSAIDs are generally effective at decreasing the rate of postoperative CME.
 |
|
SCENARIO 4. Patients with pseudoexfoliation syndrome (shown here) may be candidates for a MIGS procedure. This image was originally published in the ASRS Retina Image Bank. Jason S. Calhoun. Pseudoexfoliation Syndrome. Retina Image Bank. 2015; Image Number 25758. © The American Society of Retina Specialists.
|
SCENARIO 5
Toric IOL Planning and Alignment
WARREN E. HILL, MD
1. Obtain a topographic axial curvature map. This is the starting point from which we review the anterior corneal power distribution within the central 4 mm. Changes in the peripheral cornea are less important for making decisions regarding the toric IOL.
2. Confirm that the patient is a suitable toric IOL candidate. Here we are looking to confirm that the anterior corneal astigmatism is both regular and symmetrical. Regular astigmatism is demonstrated by the ability to draw a line through the center of each of the two astigmatic lobes and the corneal vertex. Symmetrical astigmatism is present when the astigmatic power on either side of the corneal vertex is similar.
3. Manually determine the orientation of the steep meridian. If the anterior corneal astigmatism is regular and symmetrical, the steep meridian for toric IOL planning can be determined by noting where a line drawn through the corneal vertex and the center of the two astigmatic lobes intersects the peripheral axis scale. By definition, this is the orientation of the steep meridian.
4. Confirm that the steep meridian on autokeratometry agrees with that which was manually determined. This confirmation is important because autokeratometry does not always provide the correct information. Because astigmatism has both magnitude (how much) and direction (which meridian), an angular error in the orientation of the toric IOL can be responsible for a large refractive surprise. Being on either side of the correct meridian by 15 degrees will reduce the astigmatic correction by approximately 50%.
5. Validate magnitude of astigmatism by autokeratometry. When the steep meridian by autokeratometry lines up with what we know to be correct from a topographic axial curvature map, we have validated the power difference between the principal meridians, and we are ready to enter this information into the toric IOL calculator of our choice. The topographic axial curvature map is our primary instrument for determining the steep meridian and the supporting instrument for the power difference. Autokeratometry is our primary instrument for determining the power difference and the supporting instrument for the steep meridian.
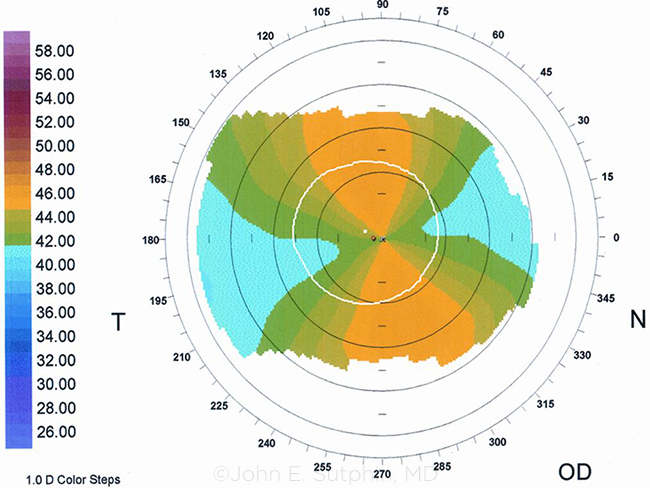 |
|
SCENARIO 5. Patients with regular and symmetrical astigmatism (shown here) are suitable candidates for toric IOLs.
|
SCENARIO 6
Patient Selection and Education for Advanced Technology IOLs
RICHARD TIPPERMAN, MD
1. Take the time to look at the patient’s placido disc mires. They contain a wealth of information and are helpful in diagnosing subtle dry eye problems as well as anterior basement membrane dystrophy. These conditions can cause reduced vision with both basic and advanced technology IOLs.
2. Educate strategically. When educating patients about the benefits of presbyopia-correcting IOLs, make the right comparison. Don’t start with the phrase “functioning visually without ever wearing glasses”—instead, start with describing what vision is like with a basic monofocal IOL and then discuss the need for spectacles for all visual tasks, from those conducted at arms’ length to those at near, such as reading.
In addition, when describing the potential for glare and halo with advanced technology IOLs, remember that “a picture is worth a thousand words.” The terms glare and halo mean different things to different patients, so it can be helpful to show the patient an example of a nighttime scene in which lights and headlights may be enlarged or have rings and to point out that the remainder of the imagery is in focus.
3. Remember that your verbiage matters. Use the term “basic IOL” to describe monofocal cataract surgery. If you use the terms “routine, standard, or conventional,” you can imply that this is the best option for all patients because it’s the one most people will choose. Use the term “advanced technology” rather than “premium” IOL. A premium product implies a luxury item—such as a fancy watch—that may be expensive but in reality doesn’t necessarily function or tell time better than an inexpensive or “nonpremium” watch. In contrast, an advanced technology product has features or benefits that are better than those available in a basic product.
4. Remember DCO. This stands for “David Chang observation.” Dr. Chang was the first person I heard discuss the importance of being alert to a sense of entitlement in a patient, as this is a red flag. These are the patients who feel that the rules do not apply to them—e.g., “I’m not filling out any paperwork until I speak with the doctor” or “I’m not having a technician do my measurements.” Postoperatively, these patients can be very challenging, as they don’t feel that they need to follow directions or comply with office protocols.
5. Manage post-op expectations. Remind patients that near vision takes longer to improve than does distance vision with a presbyopia-correcting IOL. Some surgeons tell patients not to expect easy reading vision until they are at least a month out from surgery in both eyes. This sets the patient up for success, since almost all will do dramatically better than this. With this strategy, the patient comes in for an early post-op visit with the mindset of “Hey doc, I’m already doing better than you said I would!” instead of “Doc, reading is still harder than I’d like.”
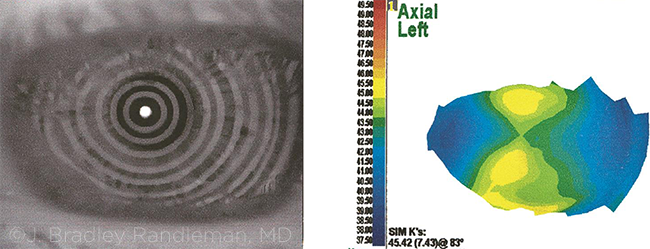 |
|
SCENARIO 6. The patient’s placido disc mires (left) are often overlooked but can provide valuable information.
|
SCENARIO 7
Success With the Light Adjustable Lens
JOHN P. BERDAHL, MD
1. Ensure that the pupil size is greater than 7 mm. The optic of the Light Adjustable Lens (LAL; RxSight) is 6 mm in diameter. Because the lens can be decentered compared to the dilated pupil preoperatively, we make sure that the pupil dilates to greater than 7 mm. If a patient is experiencing dilation fatigue after cataract surgery from multiple dilations, using 10% phenylephrine or giving the person more days between treatments can improve pupil size.
2. Emphasize the need to wear goggles. Make it absolutely clear to patients that they must wear their UV-blocking goggles before the prescription is locked in. We give them a little rubber wrist band that says “The Power of Light” to remind them to always have their goggles on.
3. Check for photosensitizing medications. There is a long list of photosensitizing medications, but the most important one is hydrochlorothiazide, a diuretic commonly used to treat hypertension. Although we do not know if hydrochlorothiazide would cause a problem if the patient were to receive a light treatment while on the medication, we stop the drug at least one week prior to delivering a light treatment.
4. Adjust both eyes the same day. We routinely provide light treatment to both eyes on the same day. The patients’ eyes are dilated, but they can see well, and the same-day appointment cuts the number of return visits in half.
5. Trial the monovision near point. Postoperatively, since the LAL is adjustable, we can determine the location of the ideal near point for monovision. Frequently we will give a patient a contact lens trial to determine if they are happy with their near point for their daily activities. We encourage them to really experience their vision in challenging situations, such as reading in dim light and driving at night. This allows us to balance anisometropia, the requirements of night-time driving, and the ability to get a near point as close as possible.
SECOND PANEL. In the second Cataract Spotlight session (Spo4V), Dr. Fram (top right) discussed surgical pearls with (clockwise, from top left) Bonnie An Henderson, MD, Kendall E. Donaldson, MD, Thomas Kohnen, MD, PhD, and Stephen G. Slade, MD.
SCENARIO 8
When and How to Perform an IOL Exchange
ZAINA N. AL-MOHTASEB, MD
1. Avoid it or wait three to six months. When a patient is dissatisfied with a premium lens, it is important to do a good history to understand the root cause. Then, you should do a good refraction, slit-lamp exam, and ocular surface evaluation to search for remnant refractive error, ocular surface disease, and other ocular pathologies. Topography and optical coherence tomography scanning of the maculae are helpful.
Next, the surgeon should treat the cause of the dissatisfaction and wait three to six months for the patient’s neuroadaptation to the lens. If the patient is still unhappy and wants an IOL exchange, doing it at this point is typically fine.
If the root cause was a refractive target miss with a toric IOL, I typically do the IOL exchange (or rotation) between one to three weeks after the original surgery.
2. Use plenty of OVD. Be generous with your use of an ophthalmic viscoelastic device (OVD) throughout the procedure. I typically use the Palay cannula and an OVD syringe to initiate the opening of the capsule. Make sure to fill the anterior chamber first, then the bag, and viscodissect around the optic haptic junction, as significant fibrosis can be present there. Also, make sure to inject a lot of OVD above and below the lens before cutting the lens in the anterior chamber.
3. Avoid iatrogenic zonular loss. After viscodissection, in order to avoid iatrogenic zonular loss, use a Sinskey hook to lift up the lens instead of rotating it. If you find significant fibrosis of the haptics to the capsule, I recommend cutting the haptics and leaving them in the bag to avoid the risk of further zonular loss by trying to remove them.
4. Lift the lens up into the anterior chamber and stabilize the IOL while cutting. There are many ways to remove the lens from the anterior chamber, including folding, but I like using the MST scissors and forceps to stabilize the lens and cut. Then, using the forceps, I remove the two pieces one at a time. If a patient previously underwent an Nd:YAG capsulotomy, the surgeon can place the new lens underneath the old lens prior to cutting, which can prevent dropping a piece of the lens.
5. Place the lens in the bag or in the sulcus with optic capture. To ensure stability of the lens and a good refractive outcome, I would recommend placing the lens in the bag if possible. If the patient previously underwent an Nd:YAG capsulotomy, then—after a good anterior vitrectomy—the IOL can be placed in the sulcus with optic capture as long as the anterior capsule is curvilinear and properly sized. If the anterior capsule is too large for optic capture, the procedure can be performed in the posterior capsulotomy.
Financial Disclosures
Zaina N. Al-Mohtaseb, MD: Alcon: C; Bausch + Lomb: C; Carl Zeiss: C; CorneaGen: C; Johnson & Johnson: C; Ocular Therapeutix: C. John P. Berdahl, MD: Aerie: C; Aerpio: C; Alcon: C; Allergan: C,L; Aurea: C,O; Avedro: C; Bausch + Lomb: C; Calhoun Vision: C; ClarVista: C; DigiSight: C,O; Envisia: C; Equinox: C,O; Glaukos: C,O; Imprimis: C,P; iRenix: C; Johnson & Johnson: C; Ocular Surgical Data: C,O; Ocular Therapeutix: C; Omega Ophthalmics: C,O; Oyster Point: C,O; RxSight: C; Vitalase: C; Vittamed: C. David F. Chang, MD: Carl Zeiss: C; Eyenovia: O; ForSight Vision 6: C,O; Ivantis: C,O; JelliSee: C,O; Johnson & Johnson: C; Long Bridge: C,O; Mynosis: C,O; Perfect Vision: C,O; Presbyopia Therapies: O; RxSight: C,O; Slack: P; Surface: O; Versant Ventures: O; Viewpoint: C,O; Visionary Ventures II: O. Jessica B. Ciralsky, MD: Bruder Healthcare: C. Warren E. Hill, MD: Alcon: C; Carl Zeiss: C; Haag-Streit: C,P; Lensar: C; Omega Ophthalmics: C; Optos: L. Rudy M.M.A. Nuijts, MD, PhD: Alcon: C,L,S; Carl Zeiss: S; Johnson & Johnson: S; Ophtec: L. Douglas J. Rhee, MD: Aerie: C,L,O; Alcon: C; Allergan: C,S; Bausch + Lomb: L; Glaukos: S; Ivantis: C,L,S; Ocular Therapeutix: C. Neal H. Shorstein, MD: None. Richard Tipperman, MD: Alcon: C.
See the disclosure key at www.aao.org/eyenet/disclosures.
|