By Gabrielle Weiner, Contributing Writer, interviewing Anthony J. Aldave, MD, Jonathan H. Lass, MD, and Jennifer Y. Li, MD
Download PDF
If you perform endothelial keratoplasty, are you comfortable accepting cornea tissue that’s 10 or 11 days old? Although the FDA has approved hypothermic storage of donor cornea tissue for up to 14 days, most U.S. surgeons are offered tissue that is three to seven days old because of the typical surplus of cornea tissue in this country.
However, the future of that surplus is uncertain. The U.S. population is aging, and there is a concomitant increase in demand for cornea tissue, noted Jonathan H. Lass, MD, at Case Western Reserve University School of Medicine and the University Hospitals Eye Institute in Cleveland, Ohio. In addition, the donor pool is at greater risk from emerging infections—and the current opioid epidemic means eye banks need more time to screen donors.
Extending storage time gives eye banks greater flexibility to evaluate and distribute donor corneas, significantly expanding supply. But U.S. surgeons have become so accustomed to the current practice of shorter-term storage that “many are reluctant to go beyond seven days should the need arise,” said Dr. Lass, who also served as chair of the Cornea Preservation Time Study (CPTS).
 |
|
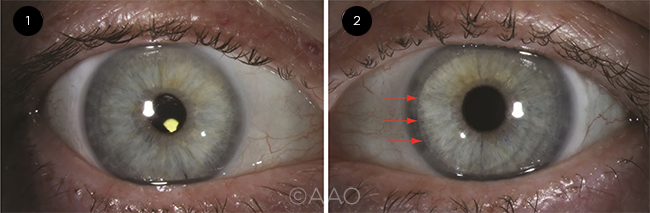
COMPARISON. These images are from the same patient. (1) Right eye, two months after DMEK (Descemet membrane endothelial keratoplasty). Best-corrected visual acuity (BCVA) was 20/20; the edges of the graft are not visible on direct diffuse illumination. (2) Left eye, eight months after DSAEK. BCVA was 20/25; the nasal graft edge is visible (red arrows).
|
Evidence From the CPTS
For evidence-based guidance on storage times, cornea surgeons can turn to the CPTS for assurance.
Question of preservation time. The NEI-sponsored CPTS was the first to study whether endothelial keratoplasty using donor corneas preserved for eight to 14 days could be as successful as surgery with donor corneas that had been preserved for up to seven days.
The CPTS enrolled 1,330 study eyes that underwent Descemet stripping automated endothelial keratoplasty (DSAEK) for corneal conditions associated with endothelial dysfunction and moderate risk for graft failure (Fuchs dystrophy or pseudophakic/aphakic corneal edema [PACE]).1
The objective of the CPTS was to provide scientific evidence regarding best practices for preservation time and usage. The noninferiority design rigorously demonstrated that donor corneas can be preserved for up to 11 days and still have a greater than 90% probability of graft success at three years.1 Data on endothelial cell loss at three years mirrored these findings.2
“A big question in eye banking has been the optimal timing for using tissue,” said Jennifer Y. Li, MD, at the University of California, Davis. “The CPTS gives us an evidence-based approach to deal with misperceptions about the ideal ‘freshness’ of donor corneas and should increase our eye banks’ ability to place tissue,” said Dr. Li, who also holds an advisory position with the Eye Bank Association of America (EBAA).
Of note, the three-year success rates were still high in the 12- to 14-day preservation time group (89.3%, versus 94.1% in the one- to 11-day group).1 When logistics dictate, surgeons should be encouraged to accept corneas stored 12 to 14 days, Dr. Lass said. “The minimal reduction in survival translates to a clinically acceptable level.”
Impact on surgeons’ attitudes. “As with any scientific discovery, disseminating the findings of the CPTS and influencing practice patterns will take some time,” said Anthony J. Aldave, MD, at the Stein Eye Institute in Los Angeles. “The effort to influence or change surgeon criteria for donor tissue begins at the cornea fellowship level because it is easier to shape practice patterns as opposed to changing them.”
The CPTS appears to have had an impact on the preservation time that surgeons say they are willing to accept.3 However, in practice, it hasn’t been fully tested, given the domestic supply. “Now, as eye banks ramp up in the shadow of COVID-19, we might see more corneas offered that will be over seven days old at the time of surgery. That will show if surgeons are truly comfortable accepting tissue with longer storage times,” Dr. Aldave said.
COVID-19 Update: Impact on Eye Banks
During the first wave of the COVID-19 pandemic, 90% of eye bank business in the United States was suspended. At that time, eye banks were struggling to retain their skilled work force, Dr. Lass said. “It was unprecedented and extremely difficult.”
Tracking supply and demand. Surgical supply and demand were in sync during the early phase of the pandemic, Dr. Aldave said. “Even though many donors were being ruled out because of possible COVID, it was no major issue because demand was so low” at that time, he said.
However, demand had begun to recover by mid-June, according to data presented at the EBAA’s annual meeting:1
- At the end of March, domestic surgeries using donor tissue were at 6% of normal levels; by mid-June, this had increased to 70%.
- Internationally, surgical volumes increased from 4% of normal levels to 35% during the same time frame.
- Similarly, the use of donor tissue in teaching and research settings was at 5% of normal levels at the end of March and had risen to 39% by mid-June.
Looking ahead. As we continue to move forward, “demand will exceed the normal level because of the need to clear the backlog of cases,” Dr. Aldave said. “We have to be ready for this by safely increasing the recovery and distribution of donor tissue.”
“Our goal is that donor tissue criteria will be stringent enough to maintain the safety of our supply while balancing the needs of surgeons here in the United States,” Dr. Li said. “Eye banks have been very conservative about making sure cornea tissue is safe. We are carefully monitoring the situation, making [real-time] adjustments based on data or the lack thereof.”
___________________________
1 Drury D. Eye donation and transplantation update: Current snapshot and future outlook. Presented at: EBAA Annual Meeting; June 18, 2020; Dallas.
|
Predicting Graft Success
The CPTS was also designed to study the effect of factors other than preservation time on DSAEK outcomes. It prospectively tracked over 50 factors that might impact graft success or failure and endothelial cell loss three years after surgery.4
Risk: diabetic donors. The most remarkable finding, according to Dr. Lass, was that diabetes in the donor correlated with lower graft success and greater endothelial cell loss at three years, as well as more graft dislocations overall, particularly among patients who experienced primary or early graft failures.1,2,4 “Prior to the CPTS, there were conflicting studies on whether diabetes in the donor could affect transplant success and cell loss,” said Dr. Lass.
Going forward, researchers need to define and study disease severity in donors (e.g., from prediabetes to diabetic nephropathy, neuropathy, and peripheral vascular disease). “Given that this country has an epidemic of diabetes,” Dr. Lass said, “we need to establish” whether tissue from particular subsets of diabetic donors can be used.
Risk: recipients with PACE. Failure was more likely in PACE recipients than in recipients with Fuchs dystrophy, with a significant difference in late failures, but not in primary/early failures.4 This may be due to the PACE group having a lower peripheral endothelial cell reserve than the Fuchs group, said Dr. Lass, who noted that further study is needed.
Risk: operative complications. By far the strongest predictor of failure was operative complications. These included an inverted graft, unplanned vitreous loss, posterior capsule rupture, and significant hyphema. They also comprised difficulty with unfolding and positioning tissue with/without use of a positioning hook, a difficult air fill and retention in positioning, and/or reinsertion of the donor tissue after extrusion.4 “The most important thing to focus on for your patients is minimizing iatrogenic donor tissue damage by any means possible,” said Dr. Aldave.
Not a risk: donor age. Aligning with prior findings from the Cornea Donor Study on penetrating keratoplasty (PK), CPTS found no evidence to suggest that advanced donor age is correlated with DSAEK survival.5
Not a risk: additional factors. In addition, no evidence suggests that preoperative donor endothelial cell density or donor DSAEK diameter is associated with graft survival, according to Dr. Aldave.
Dr. Aldave further noted that outcomes were not affected by donor gender, race/ethnicity, or cause of death. In addition, they were not affected by death to preservation time, time from dissection to surgery, gender mismatch, or type of injector used.
Unclear: lenticule thickness. In the CPTS report on graft dislocations, much of the data mirrored the reports on graft success and endothelial cell loss.3 Operative complications and diabetes in the donor were two of three predictors of failure. But the third factor—one that Dr. Aldave called a mystery—involved lenticule thickness. That is, a donor cornea with thicker precut thickness, despite the postcut lenticule thickness (ranging in eyes from 14% under 100 μm to 31% over 150 μm), was more likely to be associated with complications.6
“Much focus has been placed on lenticule thickness and graft dislocation, but why, if the eye bank is able to prepare lenticules of a given thickness from donors of variable thicknesses using different microkeratome heads and preparation techniques, should the original thickness matter?” Dr. Aldave asked.
Additional nuances regarding graft rejection. The only statistically significant factor associated with graft rejection was recipient age. Older DSAEK recipients had a lower rejection risk than younger recipients (defined as those younger than age 50), Dr. Lass said.
Factors identified by two major PK studies (the Cornea Donor Study and the U.K. Registry Study) as being associated with a higher risk of rejection—gender, gender mismatch, prior use of glaucoma medications, and a history of glaucoma surgery—were not found to be significant with DSAEK.5,7
Antifungal Supplementation? Not Yet
An increasing incidence of fungal infection following endothelial keratoplasty has stimulated discussion in eye bank meetings about whether to pursue antifungal supplementation of donor storage media. “Though fungal infection is still uncommon, the incidence is trending upward, and it is devastating for patients when it occurs,” Dr. Li said.
At this point, there is some support for the hypothesis that antifungal supplementation with a number of different antifungal agents does reduce the growth of fungi, usually Candida, in storage media, Dr. Aldave said. However, study findings on the safety and efficacy of antifungal supplementation are inconsistent, he noted. Problems include variations in study designs, Candida species tested, choice of antifungal agents and concentrations, and duration of exposure of the cornea to the antifungal agent.
Although the CPTS showed that you can use cornea tissue that is 12-14 days old, “can an antifungal agent be in a solution for that long without damaging the tissue?” Dr. Aldave asked. (In the CPTS, two cases of fungal infections occurred among the 1,330 study eyes, but there was no statistically significant difference in terms of infections between the two preservation times. In addition, with regard to rim cultures, no difference emerged between the two preservation times.1)
Going forward, researchers are working on finding the optimal way to confer maximum protection with minimal toxicity.
___________________________
1 Mian SI et al. Cornea. 2018;37(9):1102-1109.
|
Practical Advice
When discussing the freshness and safety of donor tissue, Dr. Li emphasized that EBAA-accredited eye banks require donor tissue to meet a rigorous standard of quality and safety. In her personal practice, she has never questioned or sent back a donor cornea to her eye bank. “Accreditation is not just a rubber stamp. Standards are updated on a regular basis, including amidst the COVID-19 pandemic. As long as a surgeon works with an accredited eye bank, freshness and safety of donor tissue are not factors he or she should need to think about.”
Thus, she and Drs. Aldave and Lass said, surgeons should worry less about donor tissue quality and more about minimizing operative complications. They offered the following suggestions:
Patient age. Surgeons should carefully monitor patients younger than age 50 because of their higher risk for rejection, Dr. Lass said.
Other considerations. In addition, it is prudent to be cautious with patients who have had prior glaucoma surgery, Dr. Li said. (Only 31 eyes in the CPTS had a history of glaucoma surgery, and eyes with previous tube shunts were not included. Though not statistically significant, eyes with prior glaucoma surgery had a lower graft success rate.3 As noted above, in PK studies, prior glaucoma surgery was associated with a higher risk of rejection.7)
Dr. Aldave added, “For eyes at highest risk for donor detachment, rejection and/or failure, such as hypotonous eyes or those with prior glaucoma surgery, maybe it would be justified to request tissue from a nondiabetic donor whose corneas are of normal thickness. I’ll probably get a lot of flak for suggesting that, but these are the only factors within the surgeon’s control besides operative skills.”
___________________________
1 Rosenwasser GO et al. JAMA Ophthalmol. 2017;135(12):1401-1409.
2 Lass JH et al. JAMA Ophthalmol. 2017;135(12):1394-1400.
3 Hannush SB et al. International Journal of Eye Banking. 2018;6:1-12.
4 Terry MA et al. Ophthalmology. 2018;125(11):1700-1709.
5 Mannis MJ et al., for the Writing Committee for the Cornea Donor Study Research Group. Ophthalmology. 2013;120(12):2419-2427.
6 Aldave AJ et al. Am J Ophthalmol. 2019;203:78-88.
7 Stulting RD et al. Am J Ophthalmol. 2018;196:197-207.
___________________________
Dr. Aldave is professor of ophthalmology, Walton Li Chair in Cornea and Uveitis, chief of the Cornea and Uveitis Division, and director of the Cornea and Refractive Surgery Fellowship at The Jules Stein Eye Institute in Los Angeles. He is also chair of the Policy Position and Review Committee for the EBAA in Washington, D.C. Relevant financial disclosures: None.
Dr. Lass is Charles I. Thomas Professor and Vice Chair for Academic Affairs in the Department of Ophthalmology and Visual Sciences at Case Western Reserve University in Cleveland, Ohio. He is also a member of the Center for Anterior Segment Diseases and Surgery at the University Hospitals (UH) Eye Institute, medical director of the UH Cornea Image Analysis Reading Center, and medical director of Eversight Ohio. Relevant financial disclosures: NEI: S.
Dr. Li is professor of ophthalmology and chief of Cornea and External Disease at the University of California, Davis. She is also chair of the Medical Advisory Board for the EBAA in Washington, D.C. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Aldave American Academy of Ophthalmology: L; Avellino Laboratories: C; ClearView Healthcare Partners: C; Cornea: L; Dompé: C; EBAA: S; Evolve Medical Education: L; Eyevance: C; GlaxoSmithKline: C; Guidepoint: C; Hospital Authority of Hong Kong: L; NEI: S,L; Petrichor Healthcare Capital Management: C; Tissue Aegis: C; U.S. Department of Defense: S; Wills Eye Hospital: L; W.L. Gore & Associates: C.
Dr. Li None.
Dr. Lass Dr. Lass is medical director of the University Hospitals Cornea Image Analysis Reading Center (CIARC), which receives contracted funding for the following corporate work: Alcon/Novartis; Allergan; Duke Eye Center; Envisia; Eyetech; Glaukos; InnFocus; Ivantis; Kowa; PolyActiva; Presbia; Trefoil; Trial Runners; Valeant; VisionCare.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|