By Annie Stuart, Contributing Writer, interviewing Emily Y. Chew, MD, Sunir J. Garg, MD, and Eugene Yu-Chuan Kang, MD
Download PDF
Today’s ophthalmologists have good treatment options for the advanced stages of diabetic retinopathy (DR)—laser, intravitreal injection, and vitrectomy—said Eugene Yu-Chuan Kang, MD, at the Chang Gung Memorial Hospital in Taiwan.
However, these treatments can be onerous, invasive, or costly. “We need something with few side effects that’s inexpensive and can be given widely,” noted Emily Y. Chew, MD, at the NEI.
Statins and fibrates to the rescue? Could lipid-lowering drugs such as statins and fibrates fit the bill? Certainly, they are widely available and cost-effective. “You can buy a lifetime supply of simvastatin or fenofibrate for the cost of one vial of our intravitreal injections,” said Sunir J. Garg, MD, at Wills Eye Hospital in Philadelphia. They do carry a risk of side effects, however (see “Caution: Drug Side Effects”).
Statins and fibrates are back in the spotlight, thanks to a recent retrospective study conducted in Taiwan by Drs. Garg and Kang and their coauthors. They investigated the association between statin therapy and the development of DR in patients with diabetes and dyslipidemia and found that statin use was associated with a decreased prevalence of diabetic retinopathy.1
 |
|
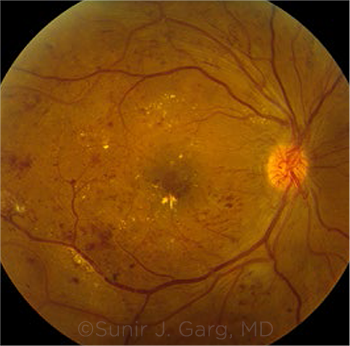
PROGRESSION. Neovascularization of the optic disc along with hard exudates and scattered retinal hemorrhages, seen in a patient with proliferative DR and DME. Some evidence suggests that lipid-lowering medications slow DR progression.
|
Laying the Groundwork
Statins help lower low-density lipoprotein (LDL), a culprit in the development of atherosclerosis, and the drugs are already prescribed for many patients with diabetes, said Dr. Chew. Fenofibrate—which is sometimes prescribed to patients who can’t take statins—also can lower LDL.
Moreover, both fibrates and statins may have multipronged effects beyond lipid control, said Dr. Kang. “These medications may also influence inflammation, angiogenesis, and cell survival.”2-4
Interestingly, the FIELD and ACCORD-EYE studies did not confirm the effect of fenofibrate on DR to be through lipid lowering, Dr. Chew said. “We don’t actually know the mechanism of action for fenofibrate in DR.”
Making the case. “Years before we had statins, we saw a good correlation between lipids and eye disease in the Early Treatment Diabetic Retinopathy Study (ETDRS),” Dr. Chew said. “The higher the lipids, the higher the risk of retinopathy.” With the advent of better lipid control, ophthalmologists are less likely to see eyes filled with hard exudates, she added.
The Wisconsin Epidemiology Study of Diabetic Retinopathy demonstrated that people with diabetes and visible lipid deposits in their retinas had higher serum lipid levels. It also found that increasing DR severity and hard exudates corresponded with increasing serum cholesterol, Dr. Garg said.
Other studies have indicated that lipid-lowering medicines have an effect on DR, said Dr. Garg, but it’s hard to tease out the size and scope of that effect. “Does it prevent diabetic eye disease? Most of the studies right now say no,” he said. “Is it useful in preventing preexisting diabetic eye disease from getting worse? Potentially.”
Approved in Australia. Although no country has approved the use of statins for DR, said Dr. Kang, Australia approved fenofibrate for this indication in patients with type 2 diabetes in 2013, following completion of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study5 and the Action to Control Cardiovascular Risk in Diabetes-Eye (ACCORD-EYE) Study6—two randomized clinical trials that showed fenofibrate to be beneficial in the treatment of DR. Thus far, however, no other countries have followed suit.
Three Key Studies
FIELD. This study, published in 2007, examined the effects of fenofibrate versus placebo in 9,795 patients with type 2 diabetes. The researchers found that, in addition to slowing progression of preexisting DR, 200 mg of fenofibrate per day reduced the occurrence of diabetic macular edema, Dr. Garg said.
Moreover, use of laser was reduced by nearly a third in these patients, said Dr. Chew. “In fact, all microvascular disease benefited, with a clear beneficial effect on the kidneys and a reduction in foot ulcers, for example.” However, fenofibrate had no effect on cardiovascular disease outcomes.
ACCORD-EYE. This study—a substudy of the parent ACCORD study—compared the results of simvastatin plus fenofibrate to those achieved with simvastatin plus placebo. The outcomes, published in 2014, were similar to those found in FIELD, said Dr. Chew, who chaired ACCORD-EYE.
Unlike in the FIELD study, however, DR was the primary microvascular outcome in ACCORD-EYE—and the addition of fenofibrate to statin therapy demonstrated benefits for the kidneys and feet, but not for the heart, she said. The benefit of fenofibrate with regard to slowing DR progression was greatest in patients with mild retinopathy.
ACCORD-EYE “found that fenofibrate plus a statin is better than a statin alone,” Dr. Kang said. Within four years, 12% of the control group progressed two or more steps on the ETDRS scale, compared with 10.6% in the statin group and 6.5% in the statin-fibrate group.6 This translated into about a one-third reduction in the progression of diabetic retinopathy.
Taiwanese study. This population-based cohort study, published earlier this year, was conducted among 37,894 Taiwanese patients with type 2 diabetes and dyslipidemia. Of these, half had taken statins.1
People taking statins were less likely to have DR, said Dr. Garg. “The study also showed that statin use was associated with fewer treatments—whether laser, injections, or surgery.” The researchers also found a greater association between a decrease in DR risk and the use of higher doses and longer duration of statin therapy, said Dr. Kang, which emphasizes the importance of medication adherence.
Challenges in Moving Forward
Nuances in disease development. One challenge with these types of studies is the difficulty of detecting changes in the diabetic eye, said Dr. Garg. This was especially true before sophisticated imaging like optical coherence tomography (OCT) became available. “In addition, if a person has no diabetic eye disease at baseline and their sugar control is reasonable, their rate of developing eye disease may not be fast,” he said. “In a two- to four-year period, we may not see a lot of difference in their disease.”
On that note, Dr. Garg pointed out that both FIELD and ACCORD-EYE showed benefit for people who already had retinopathy, but neither showed benefit for those without retinopathy at baseline. This may have been because of the length of time needed to track the impact of treatment (as well as the time needed for retinopathy to develop), Dr. Chew said.
Need for better data. “Although some of the DR data have been compelling, they are not clean and airtight enough to really hit home with your typical ophthalmologist or retina specialist,” Dr. Garg said. In addition, Drs. Garg and Kang acknowledged, their Taiwanese study is limited by its retrospective cohort design.
Need for bigger studies. What is really needed, said Dr. Garg, are larger randomized controlled trials, with sufficient power, conducted in patients with preexisting DR of various levels of severity. Ideally, too, those patients would be monitored with current imaging technologies. “But as of now, there’s not a large incentive for a big pharmaceutical company to sponsor this type of trial,” he said. “The drugs are inexpensive, and so no company is really pushing this.”
Indeed, Dr. Chew said, what’s necessary to really capture the interest and acceptance of ophthalmologists is another study conducted by ophthalmologists to test fenofibrate for this disease. A previous attempt to initiate such a trial by the Diabetic Retinopathy Clinical Research Network (DRCR.net) fell by the wayside, she said. “We’re hoping it may yet happen,” she said, explaining that such a trial “would have the potential to engage 150 clinics in the network and hundreds or thousands of patients.”
Caution: Drug Side Effects
Both fibrates and statins can cause muscle aches and liver problems, Dr. Garg noted. Other side effects linked to both drugs include headaches, abdominal pain, and nausea and vomiting. In the past, there were concerns that combining fenofibrates with statins would exacerbate muscle breakdown and fatigue, said Dr. Chew. “However, in the ACCORD-EYE Study, we found very little of that. If we saw an increase in creatinine, we adjusted the dose of the drug and the numbers went back down.”
To take fenofibrate successfully, a patient needs to have regular blood work to monitor the liver and kidney, said Dr. Garg. “In cases like this, we would typically ask the cardiologist or internist to help us.” The key, said Dr. Chew, is to communicate with the doctor giving the medical care. If he or she is unwilling to do the blood work, you can do it yourself.
|
Current Recommendations
Given the evidence to date—and the questions that remain—should clinicians incorporate lipid-lowering drugs into their treatment plan for patients with diabetes?
Stay the course. “I would not recommend a change in practice at this time,” said Dr. Garg. Controlling blood sugar and blood pressure is what’s most helpful for patients, he said. These two strategies “dwarf anything else in terms of the magnitude of importance—not just for eyeballs but for overall health.”
Glucose control. Tight glucose control can reduce the risk of eye disease by as much as 70%, said Dr. Chew—and with type 2 diabetes, it can reduce its progression rate by a third.
Blood pressure control. In ACCORD- EYE, the researchers didn’t find a treatment effect with blood pressure control, Dr. Chew said. However, other studies have. For example, the United Kingdom Prospective Diabetes Study (UKPDS) showed that, after nine years of follow-up, tight blood pressure control (target pressure of <150/85 mm Hg), compared with less tight control (target pressure of <180/105 mm Hg), reduced the progression of DR and reduced the risk of vision loss by 47%.7
Lipid control. Despite the dearth of randomized trials focusing on lipids and DR, Dr. Kang recommended that ophthalmologists emphasize lipid control in patients who have diabetes. “Ophthalmologists should encourage these patients to not only have regular eye exams but also systemic evaluations, including [analysis of] serum lipid levels.”
Adherence. If medication is prescribed, it’s important that the patients, family, and doctors make sure the patient takes it regularly, Dr. Kang said. “Without medical adherence, the disease can’t be well controlled.”
Consider fenofibrate? At this time, statins are very well accepted, said Dr. Chew, and most patients with diabetes are put on them. “The main drug in question is fenofibrate, especially since studies have not shown that it helps with cardiovascular disease,” she said. “For this reason, endocrinologists and other physicians are reluctant to prescribe fenofibrate. And ophthalmologists are also reluctant to prescribe it because of the required medical monitoring.”
However, in collaboration with a patient’s internist, cardiologist, or other medical physician, Dr. Chew has prescribed fenofibrate for some of her patients with DR.
Diabetes statements. Although the Academy’s Preferred Practice Pattern on DR does not contain a specific recommendation for fenofibrate,8 the American Diabetes Association Position Statement notes that “… there are sufficient data to suggest developing collaboration between the ophthalmologists (eye care providers) and the medical physician to consider this treatment for people affected with diabetic retinopathy.”9
___________________________
1 Kang EY-C et al. JAMA Ophthalmology. 2019;137(4):363-371.
2 Noonan JE et al. Diabetes. 2013;62(12):3968-3975.
3 Ioannidou E et al. World J Diabetes. 2017;8(1):1-6.
4 Gong Y et al. EBioMedicine. 2016;13:201-211.
5 Keech AC et al. Lancet. 2007;370:1687-1697.
6 Chew EY et al. Ophthalmology. 2014;121(12):2443-2451.
7 UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703-713.
8 American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern Guidelines Diabetic Retinopathy. aao.org/ppp. Accessed Aug. 6, 2019.
9 Solomon SD et al. Diabetes Care. 2017;40:412-418.
___________________________
Dr. Chew is director of the Division of Epidemiology and Clinical Applications and the deputy clinical director at the NEI in Bethesda, Md. Relevant financial disclosures: None.
Dr. Garg is a partner with Mid Atlantic Retina, attending physician on the Retina Service at Wills Eye Hospital, and professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University in Philadelphia. Relevant financial disclosures: None.
Dr. Kang is chief ophthalmology resident at Chang Gung Memorial Hospital, Chang Gung University, in Taoyuan, Taiwan. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chew None.
Dr. Garg Aerpio: S; Allergan: S; Apellis: S; Bausch + Lomb: C; Deciphera: C; EyeGate Pharmaceuticals: S; Johnson & Johnson: C; Nextech: O; Santen: C; Topivert: C.
Dr. Kang None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|