By Anna Kozlova, MD, Chisom T. Madu, BS, Victoria S. North, MD, and Eleanore T. Kim, MD
Edited by Bennie H. Jeng, MD
Download PDF
Bell palsy (BP) is an idiopathic, unilateral facial nerve palsy of acute onset that leads to facial muscle weakness. BP accounts for approximately half of all facial nerve palsies. The etiology is not fully understood, although some studies have investigated herpes simplex virus as a possible disease trigger.1 As many patients recover at least partial muscle function, treatment is aimed at protecting the ocular surface. For persistent disease, newer therapies such as surgical facial reanimation show promise.
Epidemiology and Risk Factors
BP is a relatively rare disease with an annual incidence of 32 cases per 100,000. It is most common between the ages of 15 and 45 years,2 and younger patients have a better prognosis. Risk factors include diabetes, hypertension, respiratory disease, obesity, pregnancy, and preeclampsia. There are no significant disparities in risk or outcomes between men and women. Recurrent disease is seen in 7% of patients, usually within 1.5 years of initial onset.2
Pathophysiology
BP is thought to result from inflammation of the peripheral facial nerve (cranial nerve [CN] VII) as it exits the skull via the stylomastoid foramen. Inflammation at the level of the geniculate ganglion can lead to obstruction, ischemia, demyelination, and subsequent nerve dysfunction.3 Herpesviruses and other viruses are under investigation as causative agents, although definitive proof has not been established.1
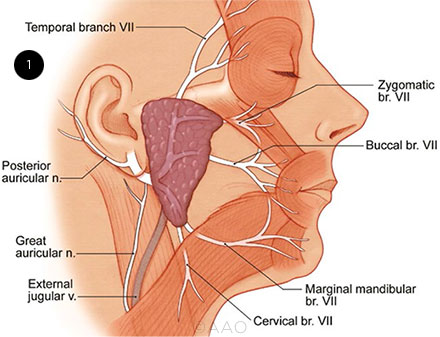
The facial nerve induces facial movement and expression via five motor branches, any of which can be affected by BP (Fig. 1):
- The temporal branch innervates the frontalis, orbicularis oculi, and corrugator supercilii muscles.
- The zygomatic branch innervates the orbicularis oculi muscle.
- The buccal branch innervates the orbicularis oris, buccinator, and zygomaticus muscles.
- The marginal mandibular branch innervates the mentalis muscle.
- The cervical branch innervates the platysma muscle.
CN VII performs several other functions. It stimulates the stapedius muscle of the ear, which protects against auditory damage by dampening vibrations in loud environments; supplies parasympathetic innervation of the lacrimal, salivary, and mucous glands; innervates the external auditory meatus, tympanic membrane, and pinna; and carries the sensation of taste from the anterior region of the tongue.
Facial nerve deficits may stem from central or peripheral causes. BP and other peripheral palsies present with ipsilateral findings affecting the upper and lower half of the face. Central lesions affect the lower contralateral side of the face, with relative sparing of the upper face due to bilateral innervation to the upper half of the face.
 |
|
ANATOMY. Illustration depicts the facial nerve branches and distribution.
|
Clinical Presentation
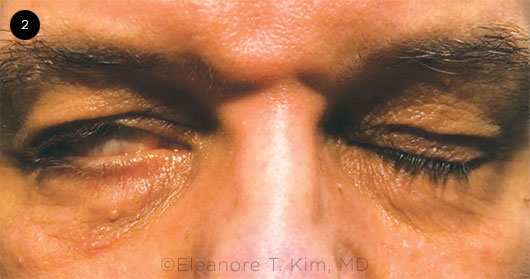
BP is characterized by unilateral weakness and partial or total paralysis of facial muscles that occurs over a period of hours to days. Clinical manifestations include facial droop, asymmetric smile, drooling, and poor eyelid closure (Fig. 2). Other symptoms include jaw pain, loss of taste, headache, and sensitivity to sound on the affected side. The overall severity of facial nerve dysfunction can be measured using the House-Brackmann grading scale4 (HBGS; see “House-Brackmann Grading Scale for Bell Palsy,” below).
Ocular-specific signs indicative of BP include widened eyelid fissure, lower eyelid ectropion, lagophthalmos, and decreased lacrimation.5 Each of these findings can contribute to ocular surface dryness and, ultimately, lead to exposure keratopathy. This is especially true in patients with an impaired Bell phenomenon (palpebral oculogyric reflex), as the inability to supraduct the eye with attempted closure further increases the risk of ocular surface disease, exposure keratopathy, and corneal ulceration.5 Neurotrophic keratopathy can manifest in cases with concomitant trigeminal (CN V) neuropathy.
Following facial nerve injury, aberrant regeneration (synkinesis) may occur. Synkinesis creates linkage between voluntary and involuntary muscle contractions, such as blinking with oral movement, hyperlacrimation, and abnormal facial/neck tightness.5 These aberrant movements may interfere with essential tasks such as chewing or swallowing.
 |
|
EYELID EFFECT. Patient has right-sided lagophthalmos secondary to Bell palsy.
|
Diagnosis
BP is a diagnosis of exclusion that is typically established with clinical findings alone. A thorough clinical history can help exclude alternate diagnoses (see “Differential Diagnosis for Bell Palsy,” below). The clinician should inquire about new medications; recent illnesses; travel to Lyme-endemic areas; pregnancy; and a history of herpes infections, inflammatory conditions, or malignancy, including prior cutaneous malignancy (perineural invasion). An accurate timeline is important for the diagnosis: Maximal weakness is typically reached within one week in BP, whereas a more gradual onset is suspicious for mass lesions.
Complete ophthalmologic examination of a patient with suspected BP includes assessment of bilateral orbicularis strength, eyelid position, lagophthalmos, and Bell phenomenon. Careful slit-lamp examination of the ocular surface is crucial. It is important to be aware that several features of the ocular exam, including motility, pupil examination, and trigeminal nerve sensation (including corneal sensation), may reveal deficits of multiple cranial nerves. Such findings should prompt critical consideration of other diagnoses such as stroke or tumors.
The clinician should also assess for facial rashes, mass lesions, and gross auditory abnormalities that could point to alternative causes. The auditory canal should be examined for herpes zoster lesions, which support a diagnosis of Ramsay Hunt syndrome.
Basic laboratory studies can be obtained to establish a baseline evaluation and to rule out other inflammatory or infectious causes of facial nerve palsy (e.g., complete blood count, erythrocyte sedimentation rate, C-reactive protein, Lyme antibody, syphilis screen). If the physical exam or reported history is suspicious for other etiologies, additional blood work or imaging may be useful.
Imaging and electrodiagnostic testing. When examination suggests a central source or when the timeline or additional clinical features argue against acute idiopathic facial nerve palsy, neuroimaging is indicated. Magnetic resonance imaging (MRI) of the brain and orbits, with and without contrast, effectively highlights the soft tissues of the cranial nerves and associated parenchyma. A dedicated facial nerve MRI can be helpful when perineural invasion or an inflammatory process is suspected. Computed tomography scans can identify bony fragments impinging on CN VII in cases of trauma.
Electrodiagnostic testing modalities such as electromyography (EMG) and electroneurography (ENoG) can be useful in patients with severe BP.6 These tests measure electrical activity of a muscle or nerve to quantify the extent of nerve damage. Ideally, they are performed seven days after symptom onset, when Wallerian degeneration of the nerve is optimally measured. A 90% or greater loss in ENoG or EMG signal amplitude indicates a low likelihood of spontaneous recovery and may help identify patients who would benefit from facial nerve decompression surgery.3
House-Brackmann Grading Scale for Bell Palsy
|
GRADE
|
CHARACTERISTICS
|
|
I
|
Normal facial function in all areas.
|
|
II
|
Gross: Slight weakness noticeable on close inspection; may have very slight synkinesis.
At rest: Normal symmetry and tone.
Motion: Forehead, moderate to good function; eye, complete closure with minimum effort; mouth, slight asymmetry.
|
|
III
|
Gross: Obvious but not disfiguring difference between two sides; noticeable but not severe synkinesis, contracture, and/or hemifacial spasm.
At rest: Normal symmetry and tone.
Motion: Forehead, slight to moderate movement; eye, complete closure with effort; mouth, slightly weak with maximum effort.
|
|
IV
|
Gross: Obvious weakness and/or disfiguring asymmetry.
At rest: Normal symmetry and tone.
Motion: Forehead, none; eye, incomplete closure; mouth, asymmetric with maximum effort.
|
|
V
|
Gross: Only barely perceptible motion.
At rest: Asymmetry.
Motion: Forehead, none; eye, incomplete closure; mouth, slight movement.
|
|
VI
|
Total paralysis: No movement.
|
Prognosis
The majority of BP patients experience near-complete or complete spontaneous recovery within three weeks of onset, and nearly all patients will recover within five weeks.3 In a large study of the natural history of BP, 100% of patients achieved some degree of muscular recovery, and 71% achieved complete recovery.2 The extent of eventual recovery was associated with severity of palsy at presentation and with patient age. Among patients who had incomplete palsy, 94% recovered fully, compared with 61% of patients who had complete palsy.
The recovery time was also associated with disease severity: within two months for incomplete palsy versus three to five months for complete palsy. No patients who had residual deficits after six months achieved complete recovery. An early recovery was also associated with better final prognosis, whereas later recovery was associated with sequelae such as aberrant regeneration.2
Patients aged 5 to 14 years were found to have the most favorable prognosis, with 90% achieving full recovery. Likelihood of full recovery decreased with age, with only about one-third of patients above the age of 60 years regaining normal function.2
Differential Diagnosis for Bell Palsy
|
Infectious
|
Lyme disease, viral (e.g., herpes simplex, varicella zoster, adenovirus), bacterial otitis media
|
|
Inflammatory/Autoimmune
|
Sarcoidosis, Guillain-Barré syndrome, Hashimoto encephalopathy, multiple sclerosis
|
|
Compressive Lesions
|
Cerebellopontine angle tumors, metastatic neoplasms, benign cysts, cholesteatoma
|
|
Trauma
|
Fracture of bony fallopian canal, facial nerve lacerations, blunt force trauma, penetrating trauma
|
|
Ischemic
|
Stroke affecting vasculature supplying CN VII, atherosclerosis
|
|
Miscellaneous
|
Influenza vaccination (reported from intranasal version, since recalled)
|
|
SOURCE: Adapted from Tiemstra JD, Khatkhate N. Am Fam Physician. 2007;76(7):997-1002.
|
Medical Management
Although most patients with BP recover spontaneously, treatment can speed recovery and potentially prevent permanent sequelae. In general, patients with more severe clinical findings may benefit from more aggressive management in order to prevent permanent complications such as exposure keratopathy.
Corticosteroids. Treatment with oral steroids is associated with increased rates of complete recovery7 and should be initiated within 72 hours of symptom onset. Clinical guidelines recommend a 10-day course of oral steroids with five days at a high dose (prednisolone 50 mg/day for 10 days or prednisone 60 mg/day for five days) followed by a five-day taper.6 Caution should be taken in patients with diabetes, and close monitoring of glucose levels is essential.
Antivirals. Antiviral treatment for BP is controversial; some research shows no benefit when these drugs are added to corticosteroids.7 However, there is evidence for benefit in severe or complete BP, particularly with the use of higher-bioavailability antivirals such as valacyclovir and famciclovir.8 In one study, a dosing regimen of oral famciclovir (750 mg/day) for seven days led to increased rates of recovery.8
Ocular Surface Protection
BP can be a vision-threatening disease if persistent lagophthalmos leads to significant exposure keratopathy, corneal ulceration, or eventual neurotrophic keratopathy. It is essential to initiate measures such as the following to protect the ocular surface while facial paralysis is present5:
- Lubrication: artificial tears, ointments, moisture chambers, punctal occlusion, humidifiers.
- Temporary induced ptosis: chemodenervation with botulinum toxin, application of external gold weight.
- Temporary eyelid closure: eyelid taping or suture tarsorrhaphy.
- Corneal surface coverage: bandage contact lens.
Longer-term solutions can be considered to address persistent corneal exposure, lacrimal apparatus malfunction, aberrant regeneration, and poor cosmesis:
- Ocular surface coverage: scleral contact lens.
- Eyelid implants: upper eyelid gold or platinum weights, palpebral springs.
- Upper lid retraction repair: müllerectomy or levator recession.
- Lower lid ectropion repair: medial and/or lateral canthoplasty, wedge resection, medial canthal Royce-Johnston suture, or autologous fascial sling.
- Facilitation of drainage/tear reduction: botulinum toxin to lacrimal gland.Epiphora is multifactorial in nature in facial nerve palsy patients, due to loss of tear pump, lower eyelid malposition, reflex epiphora from exposure, and Certain cases may ultimately benefit from dacryocystorhinostomy or use of a Jones tube.
Surgical Management
Facial nerve decompression. CN VII passes through rigid bony structures that restrict expansion during inflammation, resulting in nerve ischemia and damage when inflamed. Surgical decompression relieves this structural constraint but is controversial.
A recent meta-analysis found higher rates of complete recovery in patients with complete palsy (HBGS V and VI) or severe nerve degeneration (>90% degeneration on electrodiagnostic testing) who underwent surgical decompression versus conservative management. Optimal results were seen with decompression within 14 days of symptom onset, with some possible improvement after that window.9
Facial reconstruction and reanimation. Finally, surgical static and dynamic techniques can be considered in patients with lasting damage and limited potential for recovery in order to improve functional and aesthetic outcomes.5,10 Static techniques (slings) improve resting symmetry of face without restoring movement. Dynamic techniques include the following:
- nerve interposition grafting: bridges the gap of a nerve defect;
- hypoglossal-facial nerve end-to-end anastomosis of CN XII and CN VII;
- contralateral facial nerve graft: uses donor nerve to bridge a damaged facial nerve to the contralateral (unaffected) facial nerve;
- muscle bundle transfer (e.g., temporalis muscle): employed when affected muscle is no longer viable; and
- microneurovascular free flap transfers: transplants from remote donor sites.
Aberrant regeneration can be managed with facial physical therapy for muscular weakness and synkinesis. Neurectomy and chemical denervation with botulinum toxin can be useful, especially in cases of hyperlacrimation (i.e., crocodile tears).
Recent Developments
Facial reanimation is a growing field, with research into the use of flaps or grafts from small muscles and muscle units such as the platysma to reconstruct a dynamic blink.11 There have also been advancements in the use of nerve conduits and acellular autografts. Improved harvesting and microsurgical techniques have allowed for greater success in these surgeries.
Advances have also been made in the management of neurotrophic keratopathy, which may develop with long-standing exposure. These include biopolymer drops that mimic the corneal component heparan sulfate, and coenzyme Q10 drops to suppress connexin 43 and speed epithelial healing.12 Topical cenegermin (Oxervate) has recently been found effective in the management of neurotrophic keratopathy. The active ingredient is a recombinant nerve growth factor (NGF) structurally identical to human NGF, mechanistically supporting corneal epithelial cell health and stimulating corneal reinnervation. When long-term recovery is not anticipated, corneal neurotization with nerve grafting may improve corneal structure, sensation, and function.
Conclusion
BP is an acute, idiopathic facial nerve palsy that resolves fully in the majority of patients within two months. Patients with incomplete palsy at onset have a better prognosis and a speedier recovery. Corticosteroids and, possibly, antivirals can hasten recovery and prevent long-term sequelae. Protection of the ocular surface with conservative measures or surgical eyelid repair is key to ameliorating exposure and preventing exposure keratopathy and vision loss. Other surgical strategies include decompression of the facial nerve canal and facial reanimation techniques; however, because the majority of patients recover spontaneously, these methods should be reserved to address permanent complications.
__________________________
1 Stjernquist-Desatnik A et al. Ann Otol Rhinol Laryngol. 2006;115(4):306-311.
2 Pietersen E. Acta Otolaryngol Suppl. 2002;(549):4-30.
3 Tiemstra JD, Khatkhate N. Am Fam Physician. 2007;76(7):997-1002.
4 House JW, Brackmann DE. Otolaryngol Head Neck Surg. 1985;93(2):146-147.
5 Lee V et al. Eye (Lond). 2004;18(12):1225-1234.
6 Baugh RF et al. Otolaryngol Head Neck Surg. 2013;149(3 Suppl):S1-S27.
7 Sullivan FM et al. N Engl J Med. 2007;357(16):1598-1607.
8 Lee HY et al. Am J Med. 2013;126(4):336-341.
9 Lee SY et al. Clin Exp Otorhinolaryngol. 2019;12(4):348-359.
10 Faris C, Lindsay R. Curr Opin Otolaryngol Head Neck Surg. 2013;21(4):346-352.
11Telich-Tarriba JE et al. Plast Reconstr Surg. 2020;146(4):510e-511e.
12 Dua HS et al. Prog Retin Eye Res. 2018;66:107-131.
__________________________
Dr. Kozlova is a resident, Dr. North is an oculoplastics fellow, and Dr. Kim is assistant professor of ophthalmology at New York University Grossman School of Medicine in New York; Mr. Madu is a medical student at Sophie Davis School of Biomedical Education, City University of New York School of Medicine, in New York. Financial disclosures: None.
Write an Ophthalmic Pearls Article
Interested in sharing with your colleagues the latest evidence-based information about the diagnosis and medical and/or surgical management of a specific disease entity? Write an EyeNet Pearls article!
To get started, you need:
- a topic
- a faculty advisor to review your manuscript and add his/her pearls from clinical experience. (Must be a subspecialist within the topic area.)
With the above, take the following steps:
- Email eyenet@aao.org with your proposed topic.
- After the topic has been approved, submit an outline of main points, key citations to be referenced, at least one high-resolution image, and author bios and financial disclosures.
- With the medical editor’s approval, you may write the manuscript.
A checklist and writers guidelines are available at aao.org/eyenet/write-for-us.
|