By Rebecca Taylor, interviewing Elizabeth A. Bradley, MD, Raymond S. Douglas, MD, PhD, and Jennifer A. Sivak-Callcott, MD
Download PDF
Until now, the diplopia, proptosis, and other sequelae of thyroid eye disease (TED) have been thought to be irreversible. Surgical interventions, while continually improving, haven’t typically returned patients to the level of visual function or aesthetics they experienced before disease onset.
“We can never restore them to normal with surgery,” said Jennifer A. Sivak-Callcott, MD, who practices in Morgantown, West Virginia. “We can decompress the eye, move the eye muscle insertions, and line the eyes up, but the muscles aren’t going to contract normally. Our goal in doing strabismus surgery is to stop patients from seeing double when [they are] looking straight ahead or down.”
 |
|
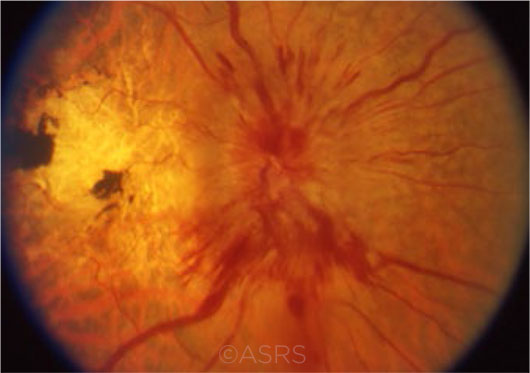
NEW ERA. Ideally, drug treatment will allow clinicians to intervene long before the occurrence of sequelae such as proptosis—or even vision loss because of compressive optic neuropathy (shown here). This image was originally published in the ASRS Retina Image Bank. Eric A. Postel, MD. Compressive optic neuropathy—thyroid eye disease. Retina Image Bank. 2012; Image Number 2862. © The American Society of Retina Specialists.
|
Breakthrough Therapy
Enter teprotumumab (Genmab/Roche), a new immunomodulatory agent that has received Fast Track, Breakthrough Therapy, and Orphan Drug designations by the FDA, based on results of a phase 2 study of patients with TED.
Reversing proptosis. In late 2017, the study, published in The New England Journal of Medicine (NEJM), reported that teprotumumab reduced and even reversed the sequelae of TED.1 “It’s the first medical treatment shown in any study to actually reduce proptosis,” said Dr. Sivak-Callcott.
“It’s very exciting,” said lead investigator Raymond S. Douglas, MD, PhD, at Cedars-Sinai Medical Center in Los Angeles. He added that the drug “is really going to change how we think about this disease from start to finish, although there aren’t yet any other clinical studies of this drug.” (A confirmatory phase 3 study of 76 patients is under way.)
Mechanism of action. With thyroid disease, the body makes antibodies against the thyroid gland. “These receptors are similar to receptors in orbital tissues, including the progenitor cells,” said Dr. Sivak-Callcott. “So in thyroid eye disease, you develop an autoantibody that attacks the thyroid and the soft tissues of the orbit.”
Teprotumumab “is designed to block the IGF-1 [insulin-like growth factor] receptor and turn this receptor into ‘stealth mode,’ so the immune system doesn’t see it,” said Dr. Douglas. “It goes right at the heart of the molecular distinction of this autoimmune disease, instead of treating downstream cytokines or other inflammatory markers. This drug, in a sense, targets the match that starts the forest fire of TED.”
Teprotumumab targets the IGF-1 receptors throughout the body, not just those in the thyroid, noted Elizabeth A. Bradley, MD, at Mayo Clinic College of Medicine and Science in Rochester, Minnesota.
Results. The phase 2 trial of teprotumumab spanned 22 centers across the United States and Europe. Of the 87 patients in the intention-to-treat group, 42 received teprotumumab, and 45 were controls. At week 24, 29 of the 42 (69%) of those who received teprotumumab had positive results, versus 9 of 45 (20%) of those given a placebo. As early as the six-week mark, 18 (43%) patients who received teprotumumab had responded positively based on a number of functional and aesthetic measures, versus 2 (4%) of those in the control group.1
Rapid response. The drug “appears to work very quickly,” said Dr. Douglas. “After two or three doses of the drug, the eye bulging and double visionimprove, and patients are ecstatic because the results are so rapid.” TED leaves a trail of devastation that doesn’t go away. But teprotumumab seems to not only reduce the inflammation, as steroids do, it also appears to reverse the underlying disease process, he said. “No other drug has reversed the damage this disease causes.”
Length of effect? Moreover, the results appear to be long lasting, Dr. Douglas said. “If patients have to be on a drug for the rest of their lives, or it only works a short time, we all become less enthusiastic. But the data show that we’re making a long-term change, with very low recurrence rates.” As he noted, this raises the possibility of eliminating the need for surgery.
Unknowns and Challenges
Issues yet to be resolved include the following:
Delivery method. Teprotumumab is delivered intravenously; how well patients will accept that as a delivery method is an unknown. “Teprotumumab is delivered via IV infusion, given every three weeks, for eight infusions,” said Dr. Douglas. “Maybe in the future we can do fewer infusions, but everyone would rather take a pill than [be given] an IV.”
Cost. “We don’t yet know the risk/benefit ratio, in terms of cost and side effects, for immunomodulatory agents,” Dr. Bradley said. “We don’t yet know what the cost of this therapy will be to [be able to] do a cost-effectiveness analysis. What cost is society willing to bear, and what risks and side effects are patients willing to bear?”
Side effects. In the NEJM study, hyperglycemia was the primary side effect associated with teprotumumab. This affected patients with diabetes and necessitated medication adjustments. Some patients also reported nausea following the first and second infusions.
“TED is not potentially life threatening, so we need more data and larger studies to determine the low-frequency, less common side effects,” Dr. Bradley noted. “The surgical risks are already known and are confined to the eye socket.”
Earlier intervention? The phase 2 trial was initially designed to study only patients diagnosed within the past nine months with moderate-to-severe TED. But an extension study, called OPTIC-X, is now planned to evaluate teprotumumab treatment in patients who are in the earliest stages of TED, Dr. Douglas said.
Diagnosing TED
Drs. Bradley, Douglas, and Sivak-Callcott all agree: Comprehensive ophthalmologists are on the front line in diagnosing TED.
Early detection. TED is more common than most people realize, said Dr. Sivak-Callcott. “Patients will come in with a complaint of watering, swelling, foreign body sensation, and redness, and those complaints can [signify] many different things, but TED is the most common disease to affect the orbit,” she said. Diagnosis may be missed, she said, because these symptoms are so similar to those of general dry eye.
Diagnostic clues. “Looking for early signs of restrictive myopathy is important because one of the most debilitating sequelae of this disease is double vision,” Dr. Sivak-Callcott said. “The eye disease develops within about 18 months of the thyroid disease, and that 18 months can precede the [actual] diagnosis of hyperthyroidism.”
“When any patient comes in with chronic irritation and redness around the eyes,” Dr. Douglas advised, ask the person how they’ve been feeling in general. Is their heart racing? Are they losing weight? Do they have anxiety or trouble sleeping? Have they had any thyroid problems?
Four tests to run. The clinician should order tests for thyroid-stimulating hormone (TSH, the most sensitive measure to detect thyroid abnormality), free T3, and free T4.
In addition, said Dr. Sivak-Callcott, it’s important to test for thyroid-stimulating immunoglobulin (TSI), a measure of autoantibodies. That’s because patients may be in a euthyroid state, in which the results of their thyroid tests are normal—“but their antibodies may be up, and they have not become hyperthyroid as of yet. It may lead you to suspect the diagnosis earlier if you remember to check for the autoantibodies.”
|
Other Research Targets
Other immunomodulatory agents that have been explored for TED include the following:
Tocilizumab (Genentech), used primarily to treat rheumatoid arthritis, “is a nice pipeline molecule, but it’s many years behind the development of teprotumumab” in terms of treating TED, said Dr. Douglas. “It might be a complementary molecule at some point, helpful in reducing inflammation. It inhibits the interleukin-6 pathway, but it’s unclear [at this point] whether it will reduce or reverse the damage of TED.”
Tocilizumab also has the potential to engender more side effects, he said, “because it dampens down the entire immune system, rather than targeting just one immune interaction—the one specific to TED.”
Rituximab (Genentech), used to treat certain autoimmune diseases and types of cancer, also has been studied for TED. To date, however, it has produced no improvement with proptosis or double vision, Dr. Douglas said.
___________________________
1 Smith TJ et al. N Engl J Med. 2017;376(18):1748-1761.
___________________________
Dr. Bradley is associate professor of ophthalmology at the Mayo Clinic College of Medicine and Science in Rochester, Minn. Financial disclosures: None.
Dr. Douglas is professor of surgery at Cedars-Sinai Medical Center in Los Angeles. He also practices in Beverly Hills, Calif., and Shanghai. Financial disclosures: NEI: S.
Dr. Sivak-Callcott is an oculofacial surgery specialist with Mon Health Medical Center in Morgantown, W. Va. Financial disclosures: None.
See the disclosure key at www.aao.org/eyenet/disclosures.