Download PDF
The cornea is an active site of research in regenerative medicine—which is moving the prospect of future treatments away from invasive and toward noninvasive procedures for the epithelium and endothelium and toward 3D-cell delivery methods for generation of stromal tissue.1
Despite great potential, much of the research has been largely confined to the laboratory or preclinical trials. Establishing standardization of safe, cost-effective therapies and end points, as well as good manufacturing protocols, have posed challenges for clinical trials, said Ula V. Jurkunas, MD, at Massachusetts Eye and Ear in Boston.
She and other experts in the field provide a look at promising strategies in corneal regeneration.
Epithelial Regeneration
Approaches to epithelial regeneration range from existing and emerging types of stem cell transplantation to techniques that regenerate or reinnervate the nerves in the subepithelium.
Limbal stem cells. Although work with corneal limbal stem cells in the basal epithelial layer isn’t new, Dr. Jurkunas and her colleagues have explored a novel way to address unilateral limbal stem cell deficiency. Sponsored by the NIH, they are currently running the first human clinical trial to evaluate an approach called cultivated autologous limbal epithelial cell (CALEC) graft.
It differs from the standard approach (conjunctival limbal autograft) because only a small amount of healthy cornea is retrieved from the donor eye, and those stem cells are expanded in the laboratory, said Dr. Jurkunas. Therefore, CALEC is performed in two separate operations. “CALEC is an improvement from previous technologies because it is manufactured with all human-grade materials without animal products.
“We take a small biopsy of corneal limbal stem cells, grow them in the laboratory on amniotic membrane for two to three weeks, then transplant them back onto the same patient’s unhealthy eye after careful dissection of the scar tissue,” she said. The newly transplanted cells create a stable cornea, as the limbal stem cells provide support to full- or partial-thickness transplants, she said.
Induced pluripotent stem (iPS) cells. iPS cells are somatic cells that have been genetically reprogrammed into an embryonic-like pluripotent state, and they can develop into any kind of human cell. At Osaka University Graduate School of Medicine in Japan, Kohji Nishida, MD, PhD, is spearheading work on iPS therapy for the corneal ocular surface.
“Last year, Prof. Nishida received approval for clinical study of epithelial sheet transplantation derived from iPS cells,” said Noriko Koizumi, MD, PhD, at Doshisha University in Japan. With Prof. Ryuhei Hayashi, Dr. Nishida’s group was the first, in 2019, to transplant a 1.2-inch sheet grown from iPS cells onto the eye of a mostly blind woman, leading to improved vision. “This technique may be very useful,” provided that iPS cells can be developed with less possibility of immunological rejection, said Prof. Koizumi.
“This approach is not a low-hanging fruit,” said Dr. Jurkunas. “Unlike limbal stem cells, which are more differentiated, these cells require much manipulation and can potentially mutate or become cancer cells.” Although it’s too early to assess the full potential of iPS-derived transplants, the approach is technically challenging and may be cost prohibitive, said Ali R. Djalilian, MD, at the University of Illinois in Chicago.
“With limbal stem cell disease, the rejection risk is high, which is why Prof. Nishida has to develop epithelial cells for a single patient from his or her own iPS cells,” said Dr. Djalilian. In general, he said, cell therapies that only work for a single patient are likely to be quite expensive. That’s why researchers are looking at ways to have “universal” iPS cell lines that would be far less immunogenic and can be applied to many patients, he said. “However, there may be a way to justify iPS for single patients if it’s far superior than any other available treatment,” he added.
Corneal neurotization. Corneal neurotization is a surgical procedure for treating corneal anesthesia related to neurotrophic keratopathy. The procedure first came on the ophthalmic scene more than 10 years ago, said Asim Ali, MD, FRCS(C), at the University of Toronto in Canada. In those days, the procedure involved a large incision and transfer of nerves from the healthy to the anesthetic side of the face. The procedure was long and technically difficult, resulting in a slow recovery. The technique was never widely adopted, but the need for a solution persisted, said Dr. Ali. “Our group came up with the idea of using smaller incisions and a nerve graft to reinnervate the cornea.”
Best candidates. Patients with permanent loss of corneal sensation and an intact facial donor sensory nerve are potential candidates for this procedure. “It is not a technique for treating an active ulceration,” said Dr. Ali. “Problems of neurotrophic keratopathy must first be resolved.”
The technique. The preoperative steps involve using Semmes-Weinstein monofilaments to map out facial sensation and plan the use of donor sensory nerves, said Dr. Ali. During the procedure, a sural nerve graft is harvested from the patient’s calf. This will be used to connect the facial donor sensory nerve to the anesthetic eye. Next, the facial donor sensory nerve is isolated and accessed for use later in the surgery. Either ipsilaterally or contralaterally, the sural nerve graft is passed underneath the brow into the subconjunctival space, exiting near the limbus. The nerve graft is then split into individual fascicles that are embedded into the peripheral cornea. Last, the other end of the sural nerve graft is connected to the donor sensory nerve.
Research results. Using this technique in patients with complete, or near complete, absence of corneal sensation, Dr. Ali and colleagues have seen significant improvement in corneal sensation in the majority of patients, as well as improved health of the ocular surface from reducing the number of epithelial defects.2
Oxervate. A recombinant nerve growth factor used to treat damage to the trigeminal nerve from neurotrophic keratitis, Oxervate (cenegermin) can also stimulate local nerve growth and improve epithelial healing, and it is used primarily when active lesions are present, said Dr. Ali. However, he pointed out, Oxervate trials have not had corneal sensation as a primary outcome and have included many patients with only subnormal, not total lack of, sensation.
The reinnervation niche. Corneal neurotization is an important surgical procedure for a specific set of patients, Dr. Jurkunas said, and Oxervate can be used a little more broadly, not just for patients who’ve lost their trigeminal nerve. “I’ve had good success with it.”
More research is needed to determine the ideal niche for each, said Dr. Ali, and clinicians may even use both techniques for certain patients. With these two approaches, he said, “We’re now able to treat a problem for which we didn’t have a good option in the past.”
Stromal Regeneration
Two areas of interest in the stroma are mesenchymal stem cells (MSCs) and bioprinting.
Mesenchymal stem cells. Present in many tissues, MSCs are multipotent adult stem cells that can self-renew into multiple tissues, said Dr. Djalilian. MSCs from a single healthy donor can potentially be expanded to up to billions of cells, providing hundreds of treatment doses, he added.
“Corneal stromal stem cells (CSSCs), which have MSC properties and are derived from the anterior limbal stroma, have been found effective not only in regenerating native-like stromal tissue, but also in supporting the epithelium,” said Dr. Djalilian. CSSCs/MSCs have been used both to dampen excessive inflammatory responses and to promote corneal stromal wound healing.3 “These cells produce factors such as exosomes, which are like small packages of [therapeutic] goodies,” said Dr. Djalilian. Researchers in India, Spain, and China have conducted phase 1 clinical trials with promising results, he said, but none of these studies included control groups.
Topical application of CSSCs to acute superficial stromal wounds has been shown to prevent the formation of scars, said Vishal Jhanji, MD, FRCOphth, at the University of Pittsburgh, describing some of the early work done by the university’s Funderburgh Laboratory. Investigators at both Pittsburgh and Chicago are moving forward with these minimally invasive allogeneic treatments.
Dr. Djalilian is conducting MSC therapy research focused on wound healing for severe eye injuries. He has been cleared by the FDA to enroll patients for a phase 1 trial sponsored by the U.S. Department of Defense. The trial will establish safety, dosing, and the ideal method for delivering the cells to the cornea, which can be done as a bandage in a mixture with fibrin glue, injection, or a combination of both, he said. Funded by the NEI, a second phase 1 clinical trial of his will begin later this year to explore the use of secreted factors from MSCs for severe surface disease and limbal stem cell deficiency.
3D bioprinting. Because it is immune-privileged and avascular, the human cornea is an ideal site for tissue engineering, said Dr. Jhanji. However, the surface curvature, complex mechanical properties, and stromal cytoarchitecture of corneal tissue are challenging to mimic.4
“Extrusion-based, three-dimensional bioprinting is a potentially promising technology for construction of a corneal model,” said Dr. Jhanji. “The biofabrication strategies offer spatial control during the manufacturing process to generate full-thickness, cell-laden, 3D corneal constructs.” Because collagen makes up the stroma, which accounts for 90% of a human cornea, this makes it a logical choice of bio-ink for creating these bioengineered corneal structures, he said.
Researchers at Newcastle University, United Kingdom, were the first to produce 3D-printed corneas made with human cells.5 “Further developments in this approach must optimize the technology and components,” said Gary Yam, PhD, at the University of Pittsburgh. “This could be expanded to manufacturing the epithelium and endothelium, despite having characteristics that are distinct from the corneal stroma.”
So far, no bioprinting approaches have been able to meet all the necessary requirements, including transparency, biomechanical stability, and cell survival rates.4 “Although nothing has been tested yet in humans,” said Dr. Djalilian, “this is a cutting-edge technology and a promising approach that will bring new treatments.”
Bioengineering Materials for Cornea Repair
Many groups are working to replace corneal tissue with bioengineered materials. To date, a range of materials have been used, including human amniotic membrane, collagen constructs, and synthetic polymers.1
Collagen. This is an active field of study for both ophthalmologists and material scientists, said Prof. Koizumi. At the Université de Montréal, for example, May Griffith, PhD, and colleagues engineered a corneal stroma based on collagen alone, said Dr. Djalilian. “But in its current version, their bioengineered corneal stroma wasn’t found to be structurally strong enough in patients. Newer iterations in large animal models look more promising.”
Gel. Researchers at Harvard have developed GelCORE, an adhesive gel that is packed with light-activated chemicals that can seal cuts or ulcers on the cornea, providing an alternative to synthetic glues, which are rough, inherently toxic, and opaque.2
Yet another bioengineering approach currently being tested is LiQD Cornea, which is a cell-free, liquid hydrogel matrix for corneal regeneration that does not require light exposure—a benefit for patients with photophobia.3
It remains to be seen which of these approaches will move into clinical testing.
___________________________
1 Fuest M et al. Bioengineering. 2020;7(3):71.
2 Sani ES et al. Science Advances. 2019;5(3).
3 McTiernan CD et al. Science Advances. 2020;6(25).
|
Endothelial Regeneration
There is a shortage of donor corneas in some parts of the world, including Asian countries, where there’s some stigma about donation, said Dr. Jurkunas. “This shortage has prompted work to regenerate the endothelial cell layer.”
In addition, said Prof. Koizumi, even where donor corneas are available, corneal transplants pose several other challenges, including risk of immunological rejection, steep surgical learning curves, and corneal endothelial cell loss after surgery.
Endothelial challenges. Unlike epithelial cells, human corneal endothelial cells (HCECs) are postmitotic and difficult to grow, said Dr. Jurkunas. “Even when you are able to replicate them, you need to ensure that they don’t turn into cells that lose their native endothelial characteristics.” She added that Shigeru Kinoshita, MD, PhD, at Kyoto Prefectural University of Medicine and his colleagues (including Prof. Koizumi) have successfully grown HCECs and made them viable for patient transplants. “This is a huge advance in the field,” said Dr. Jurkunas.
“When we began this research in 2003,” said Prof. Koizumi, “we had two challenges to overcome: the expansion of HCECs in culture and the cell-delivery method. We first studied cultivated endothelial sheet transplantation using a type I collagen carrier or frozen preserved amniotic membrane. But it was difficult to find a suitable biomaterial carrier that would keep the cornea transparent.”
That’s why she and her colleagues pivoted years ago, developing a cell injection therapy—a carrier-free transplantation of cultivated endothelial cells.
Cell injection therapy. Expanding cultured endothelial cells creates the potential to treat multiple patients with one donor cornea, said Prof. Koizumi. “In our laboratory, we now can proliferate enough cells from a pair of donor corneas to allow 250 patients to receive cell injection therapy.” Unlike the steeper learning curve of corneal transplantation, she said, this technique is relatively simple.
Adding ROCK inhibitors. In 2009, Prof. Koizumi’s team was the first to report that Rho kinase (ROCK) inhibitors increase the proliferation and adhesion of corneal endothelial cells in vitro. “This discovery led us to the concept of cell-injection therapy using a ROCK inhibitor,” she said. “It enables the carrier-free transplantation of HCECs and promotes the cell adhesion to the Descemet membrane.” Keeping the patient face down for three hours after injection also aids cellular adhesion, she said.
Efficacy and safety. “Between 2013 and 2017, we completed clinical studies involving 35 patients,” Prof. Koizumi said of work with Prof. Kinoshita, who went on to complete phase 2 and phase 3 physician-initiated trials in 2019. Of the first 11 eyes they studied, all recovered corneal endothelial function with visual improvement at 24 weeks,6 and 10 eyes maintained normal endothelial function with good vision at five years.7
“Our findings showed that cell-injection therapy is a simple, less-invasive surgery and that patients obtained good visual recovery—even in complicated cases such as when [injection was] performed after keratoplasty or glaucoma surgery,” said Prof. Koizumi. And in the event of treatment failure, cell therapy can be repeated multiple times, she added.
These endothelial cells are mature, thus lowering the risk of tumorigenesis—and the researchers have not seen any rejections among 35 cases after seven years of follow-up. Before the clinical studies in 2013 began, Prof. Koizumi said, they had examined 31 organs in monkeys using histology and polymerase chain reaction, and they detected no signs of tumor cells or inflammatory responses.
Universal therapy? To enable broader distribution, Prof. Koizumi and her team at Doshisha University have developed a method for suspending, collecting, and placing the cells into glass vials for use within 72 hours. Their goal, however, is to develop a longer-lasting vial product that can be distributed to centers outside Japan, allowing this to become a universal therapy for patients with endothelial dysfunction, she said.
Magnetic cell delivery. A company called Emmecell has developed a magnetic cell delivery nanoparticle platform for delivering cells and promoting cell adhesion and integration into the cornea.8 “This method also shows good potential, and I’m looking forward to seeing the results,” said Prof. Koizumi. The technique involves growing cells in vitro from donor corneas, magnetizing them, and injecting them into the eye, after which the patient wears a magnetic patch. In a preclinical study, magnetic HCECs integrated onto rabbits’ corneas and restored corneal transparency without detectable toxicity or other adverse effects.9 In late 2020, the FDA cleared the product for a phase 1 study to evaluate its safety and tolerability in humans with corneal edema.
iPS cells. Although not yet ready for humans, endothelial cells made from iPS cells are on the horizon, said Dr. Djalilian. “Given that rejection risk is low for endothelial disease, we are likely to have universal donors so that iPS-based cells can be mass produced for all patients. Once people figure out how to make a regular supply of endothelial cells from iPS cells, this will be how everything will be done, and we’ll stop using human donor tissue for endothelial disease. We’re not there yet, but it’s just a matter of time.”
IRIS Registry Snapshot: Limbal Stem Cell Allografts
How many limbal stem cell allografts were performed in the United States from 2013 through 2020? Verana Health conducted a study that included 360 total procedures in 311 unique patients from the Academy IRIS Registry’s statistically de-identified electronic health record data. Results show that the number of surgeries remained relatively stable, with no significant fluctuations. During the eight-year period, the rate averaged 45 per year.
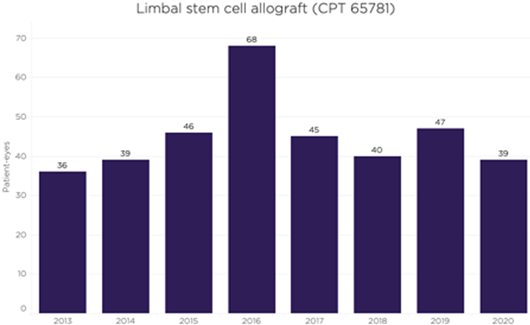
Note: The Academy has partnered with Verana Health to curate and analyze IRIS Registry data.
|
The Next Frontier
According to Dr. Jurkunas, the most exciting concept for the future is to regenerate the cornea without transplantation. Dr. Djalilian is also hopeful that researchers will learn to reprogram cells right on the eye’s surface—without removing and manipulating cells outside the eye.
“I see this as the next breakthrough,” he said. “And the more we can develop a technology that can be applied the same way to multiple patients, the more likely it can be readily implemented, making treatment available to many more patients. Anything that can fill an unmet need for patients with surface diseases will be very exciting for me.”
Small molecules and gene therapy. Researchers are attempting to enhance the function of cells10 or to reprogram them using small molecules, said Dr. Djalilian, adding that this has not yet been tested in patients. Some stromal and endothelial dystrophies have a strong genetic component, and to address this, gene therapy might be combined with cell therapy, said Prof. Koizumi. “For example, we might treat patient cells in vitro or inject a vector or chemical into the patient’s eye, treating it in vivo.”
___________________________
1 Mahdavi SS et al. Tissue Eng Regen Med. 2020;17(5):567-593.
2 Catapano J et al. Br J Ophthalmol. 2019;103(12):1724-1731.
3 Samaeekia R et al. Invest Ophthalmol Vis Sci. 2018;59:5194-5200.
4 Fuest M et al. Bioengineering. 2020;7(3):71.
5 Isaacson A et al. Exp Eye Res. 2018;173:188-193.
6 Kinoshita S et al. N Eng J Med. 2018;378:995-1003.
7 Numa K et al. Ophthalmology. 2021;128:504-514.
8 www.emmecell.com/technology.
9 Xia X et al. Invest Ophthalmol Vis Sci. 2019;60(7):2438-2448.
10 Rabiee B et al. Sci Transl Med. 2020;12(573):eaaz4894.
Meet the Experts
Asim Ali, MD, FRCS(C) Associate professor of ophthalmology and vision sciences at the University of Toronto, Canada. Relevant financial disclosures: None.
Ali R. Djalilian, MD Professor of ophthalmology at the University of Illinois College of Medicine in Chicago. Relevant financial disclosures: U.S. Department of Defense: S; NEI: S.
Vishal Jhanji, MD, FRCOphth Professor of ophthalmology at University of Pittsburgh School of Medicine in Pittsburgh. Relevant financial disclosures: None.
Ula V. Jurkunas, MD Clinician-scientist at Schepens Eye Institute of Massachusetts Eye and Ear; associate professor of ophthalmology at Harvard Medical School; and codirector at Harvard Ophthalmology Cornea Center of Excellence in Boston. Relevant financial disclosures: NEI: S.
Noriko Koizumi, MD, PhD Professor of biomedical engineering at Doshisha University and visiting professor of ophthalmology at Kyoto Prefectural University of Medicine, both in Kyoto, Japan. Relevant financial disclosures: ActualEyes: O,S,P; Senju: P,S.
Gary Yam, PhD Research associate professor of ophthalmology, corneal regeneration laboratory, University of Pittsburgh School of Medicine, Pittsburgh. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Asim Ali, MD, FRCS(C) Novartis: S; Santen: C.
Ali R. Djalilian, MD Alcon: C; Combangio: C; Dompé: C; NEI: S; Novartis: C; U.S. Department of Defense: S.
Vishal Jhanji, MD, FRCOphth None
Ula V. Jurkunas, MD Chiesi: C; Claris: C; ClearView: C; Intelia: S; Kowa: S; Kowa American Corporations: C; NEI: S; Senju: C.
Noriko Koizumi, MD, PhD ActualEyes: O,S,P; Japan Innovative Therapeutics: S; Kowa: C,S; M’s Science Corporation: C,S; Senju: P,S.
Gary Yam, PhD None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|