By Aliza Epstein, MD, Timothy Ekhlassi, MD, MPH, Lisa Faia, MD, and Evan Black, MD
Edited By: Ingrid U. Scott, MD, MPH, and Sharon Fekrat, MD
Download PDF
Giant cell arteritis (GCA), also known as temporal arteritis, is a systemic inflammatory granulomatous vasculitis that affects medium and large arteries. GCA commonly occurs in the major branches of the aorta, with a predilection for branches of the carotid artery. The disease most frequently affects individuals older than 50 years, and its incidence increases with age, peaking between 70 and 80 years. Prevalence is highest among Caucasians, and women are 2 to 3 times more likely to be affected than men.
Early diagnosis and treatment initiation are crucial, as GCA may be vision- and life-threatening. Ocular manifestations, which have been reported in up to 70% of patients with GCA,1 include arteritic anterior ischemic optic neuropathy, ocular ischemic syndrome, central or branch retinal artery occlusion, cilioretinal artery occlusion, posterior ischemic optic neuropathy, ophthalmoplegia, and ocular motor cranial nerve palsies.
Systemic manifestations of GCA include myocardial infarction, stroke, aortic aneurysm or dissection, tongue necrosis, and limb ischemia.
Etiology and Pathogenesis
The etiology of GCA is not well understood, but a combination of genetic and environmental factors is thought to play a role in its development. There have been reports of a possible relationship between GCA and a variety of viral (including varicella-zoster virus) and bacterial infections; however, these reports are not conclusive.2
In the pathogenesis of GCA, an unknown trigger activates dendritic cells within the adventitia-media border of the arterial wall. This, in turn, causes an immunological reaction leading to infiltration of T-lymphocytes, macrophages, and multinucleated giant cells into the wall.
Signs and Symptoms
The wide variability in clinical presentation makes GCA a challenging diagnosis. Systemic symptoms of GCA include headache, scalp tenderness, jaw claudication, fatigue, weight loss, fever, and polymyalgia rheumatica.
Visual symptoms include transient or permanent vision loss, diplopia, and eye pain. Of note, a subset of patients have visual changes in the absence of systemic symptoms. This condition, known as “occult GCA,” is estimated to affect 5% to 38% of GCA patients.3 In such cases, the temporal artery should be assessed on physical exam for any abnormal features, such as decreased pulse, nodularity, thickening, swelling, or tenderness.
There have been many reports in the literature investigating predictive factors for GCA. In a multicenter study of 292 patients, Duhaut et al. observed a higher frequency of visual manifestations, jaw claudication, abnormal temporal artery exam, anemia, and thrombocytosis—as well as higher levels of inflammatory markers, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)—in biopsy-proven GCA patients compared with negative-biopsy GCA patients.4
Diagnosis
In 1990, the American College of Rheumatology (ACR) published diagnostic criteria for GCA. According to the ACR recommendations, diagnosis of GCA requires at least 3 of the following 5 criteria: age ≥50 years at disease onset, new-onset localized headache, temporal artery tenderness or decreased temporal artery pulse, elevated ESR ≥50 mm/hour, and artery biopsy showing necrotizing arteritis.5 However, given reports of patients who have positive temporal artery biopsy (TAB) but do not meet the ACR criteria,6 this set of diagnostic criteria has been challenged.
Temporal artery biopsy. TAB (Fig. 1) is considered the gold standard for the diagnosis of GCA and should be scheduled as soon as possible. If clinical suspicion for GCA is high, however, corticosteroid treatment should be started immediately rather than delayed while awaiting biopsy.
TAB is a relatively straightforward procedure with infrequent complications, which can include hematoma formation and damage to the temporal branch of the facial nerve. The classic teaching is to obtain a 2-cm length specimen to avoid a false negative from “skip” areas. Some reports show that a 1-cm length may be adequate, but we recommend obtaining a longer specimen. Although it is ideal to perform TAB as early as possible, the literature suggests that a biopsy delayed for weeks to months after steroid initiation is still clinically useful (contrary to classic teaching).7
The surgeon’s intraoperative impression during TAB can provide useful preliminary information. Intraoperative characteristics suggestive of positive TAB include a thick, nodular, tortuous, pale artery with little bleeding during surgery and an apparently occluded lumen.8
Laboratory tests. The lab workup includes assessment of ESR and CRP levels, as well as a platelet count.
Elevated ESR and CRP are associated with GCA, with a sensitivity of 86.9% and 84.1%, respectively. Although these tests are nonspecific, there is a higher odds ratio of a positive TAB when both are elevated.9 However, absence of ESR and CRP elevation does not rule out GCA, and TAB should still be pursued in the setting of high clinical suspicion.
Imaging. Fluorescein angiography is useful in the workup for GCA, as it can reveal arterial occlusions and delayed or absent choroidal filling.
There has been significant interest in the use of color Doppler ultrasound as a noninvasive means of diagnosing GCA. In the hands of a skilled ultrasound technician, a hypoechoic halo around a perfused lumen is suggestive of GCA, but multiple reports have found that this technique is inferior to TAB in the diagnosis of GCA.7
High-resolution magnetic resonance imaging, magnetic resonance angiography, CT angiography, and positron emission tomography have proved to be useful diagnostic imaging modalities by revealing vasculitic changes in cranial arteries, extracranial arteries, and large vessels, including the aorta.7
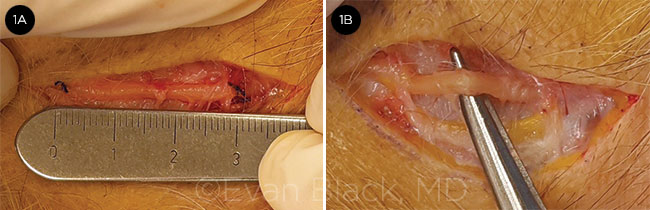 |
TEMPORAL ARTERY BIOPSY. TAB is performed in the patient from the case study, below. (1A) Measuring to ensure adequate sample length. (1B) Note the pallor and thickness of the artery, suggesting a positive specimen.
|
Treatment
The goal of GCA treatment is to prevent further visual loss and systemic sequelae of ischemia. Glucocorticoids remain the mainstay of treatment, although their long-term use is associated with significant complications, especially in elderly patients.
Steroids. Protocols for the initiation of steroids differ; some clinicians recommend starting intravenous methylprednisolone at 1 g daily for 3 days, while others begin with oral prednisone 1 mg/kg per day. Because of the risk of relapse during steroid tapering, it is essential for these patients to be followed closely by the physician managing the treatment regimen (usually a rheumatologist or an internist).
Other immunomodulators. Methotrexate, when administered with corticosteroids, can reduce relapse rates and decrease the cumulative dose of steroid therapy, but it has not been shown to reduce the rate of corticosteroid-related adverse events.
The efficacy of mycophenolate mofetil and cyclophosphamide is controversial. Newer therapeutic agents including tocilizumab, a monoclonal antibody that inhibits IL-6, have shown promising results in the treatment of GCA, but further study is needed.7
Case Study: Giant Cell Arteritis
A 78-year-old Caucasian man presented with a new complaint of a single episode of transient visual loss, lasting approximately 8 minutes, in the right eye. Exam findings, including best-corrected visual acuity (BCVA) of 20/30, as well as fluorescein angiography (FA) and optical coherence tomography, were stable compared with findings from prior visits.
Five days later, the patient returned with a new complaint of binocular diplopia. Of note, there was no afferent pupillary defect, anisocoria, or extraocular muscle restriction or tropia on exam. He was referred for lab workup.
He returned a month later with decreased BCVA of 20/400. FA was performed, demonstrating an area of choroidal nonperfusion (Fig. 2A). Lab results showed a normal ESR of 14 mm/hr, a mildly elevated CRP of 1.2 mg/dL, and a normal complete blood count. Further history revealed possible jaw claudication.
He was referred to an oculoplastics specialist for TAB, which was completed 1 day after referral. The biopsy was grossly positive (see Fig. 1)—the artery was pale and no cautery was required during the procedure, which suggests severe ischemia in the area. Histopathologic analysis of the specimen was confirmed as positive for GCA (Fig. 2B).
Although the patient was instructed to start steroid therapy before the biopsy, he did not begin the prescribed course of 60 mg of prednisone daily until immediately after biopsy. Under the direction of his rheumatologist, the patient began weekly injections of tocilizumab, and his oral steroids are still being slowly tapered 10 months after the biopsy. Although the patient’s diplopia resolved 2 months after diagnosis, his visual acuity did not improve.
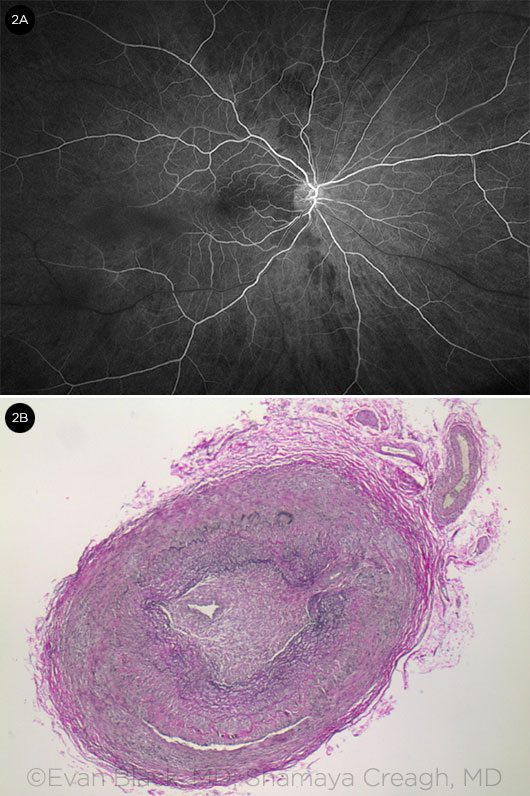
DIAGNOSTIC CLUES. (2A) Fluorescein angiography demonstrates areas of choroidal nonperfusion. (2B) Histopathology: The specimen (at 4× magnification) shows substantial thickening and cellular infiltrate of the vessel wall, confirming the diagnosis of giant cell arteritis.
|
Prognosis
The prognosis for a patient with GCA depends largely on timely recognition and treatment. Thus, clinical suspicion of giant cell arteritis must remain high on the differential diagnosis, as a delay in diagnosis and treatment initiation can lead to progressive vision loss and even binocular blindness, as well as devastating large-vessel involvement.
Compared to the general population, people with GCA have a higher mortality rate due to cardiovascular diseases in the first 2 years after diagnosis. However, mortality is not increased between 2 and 10 years after diagnosis.10
___________________________
1 Rahman W et al. Surv Ophthalmol. 2005;50(5):415-428.
2 Gilden D et al. Curr Opin Rheumatol. 2016;28(4):376-382.
3 Hayreh SS et al. Am J Ophthalmol. 1998;125(4):521-526.
4 Duhaut P et al. Ann Rheum Dis. 1999;58(6):335-341.
5 Hunder GG et al. Arthritis Rheum. 1990;33(8):1122-1128.
6 Murchison AP et al. Am J Ophthalmol. 2012;154(4):722-729.
7 Frohman L et al. Surv Ophthalmol. 2016;61(4):400-421.
8 Cetinkaya A et al. Ophthalmic Plast Reconstr Surg. 2008;24(5):372-376.
9 Kermani TA et al. Semin Arthritis Rheum. 2012;41(6):866-871.
10 Baslund B et al. Rheumatology. 2015;54(1):139-143.
___________________________
Dr. Epstein is a resident at Kresge Eye Institute, Wayne State University, Detroit. Dr. Ekhlassi is an oculoplastic surgery fellow at Kresge Eye Institute, at Consultants in Ophthalmic and Facial Plastic Surgery, Southfield, Mich., and at Oakland University William Beaumont School of Medicine, Detroit. Dr. Faia is an associate professor at Oakland University William Beaumont School of Medicine and a partner at Associated Retinal Consultants, Royal Oak, Mich. Dr. Black is a professor of ophthalmology at Oakland University William Beaumont School of Medicine, a partner at Consultants in Ophthalmic and Facial Plastic Surgery, and an associate clinical professor/oculoplastic surgery fellowship program director at Wayne State University School of Medicine. Financial disclosures (all authors): None.