Download PDF
Ophthalmologists need to continue to watch for patients who, after receiving immunotherapy for cancer, develop ophthalmic adverse effects that could prove catastrophic if they go untreated.
In a large retrospective study of patients treated with the immune checkpoint inhibitors ipilimumab (Yervoy, Bristol-Meyers-Squibb) and/or nivolumab (Opdivo, Bristol-Myers-Squibb), Yale researchers found that 15 of 1,474 patients (1%) developed ophthalmic adverse events.1
These side effects included corneal perforation, corneal punctate epithelial erosions, subconjunctival hemorrhage, uveitis, hypotony maculopathy, cystoid macular edema, serous retinal detachment, choroiditis, optic neuritis, and melanoma-associated retinopathy.
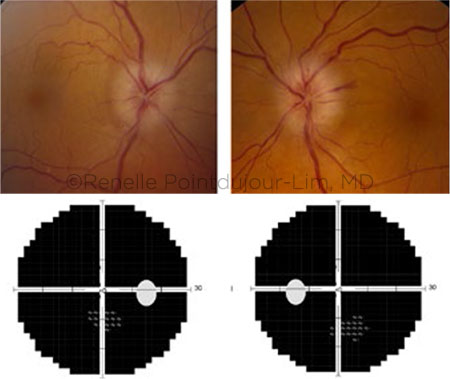 |
VF LOSS. Ocular adverse effects following treatment with immune checkpoint inhibitors included this case of profound VF loss.
|
Need for suspicion. “These adverse events are uncommon; however, we need to be aware that they can occur in patients undergoing immunotherapy,” said coauthor Renelle Pointdujour-Lim, MD, at Yale University in New Haven, Connecticut. “Even if the patient has vague nonspecific ocular symptoms, the ophthalmologist should have a high suspicion of the possibility of ophthalmic immune-related adverse events.”
Varied presentation. One challenge for clinicians is that these side effects can occur days to weeks after the infusion of immunotherapy. Moreover, they can appear mild at first but later become quite serious, Dr. Lim said. For instance:
- One patient complained of floaters that did not go away, and she was later found to have melanoma-associated retinopathy.
- Another patient was having difficulty in the periphery of her [perceived] field of vision, and her initial visual field (VF) showed nonspecific defects, Dr. Lim said. “A follow-up VF two to three months later showed profound VF loss, and she was found to have antiretinal and antioptic nerve autoantibodies.” (This case will be published in another report about the clinical spectrum of immunotherapy patients with antiretinal autoantibodies, she said.)
- A third case began as dry eye and progressed to corneal perforation by the time the patient presented to an ophthalmologist.
Growing problem. Although the incidence of ophthalmic immune-related adverse events from immunotherapy is low, the total number of cases can be expected to increase, Dr. Lim said. “The indications for use of these agents have expanded to include a broader range of malignancies, which means that more and more people will be treated with immunotherapy, and ophthalmologists need to know that these powerful agents can affect the eye,” she said.
Exam tips. “A complete examination is warranted in patients on immunotherapy, including slit-lamp and funduscopic examination,” Dr. Lim said. In addition, she said, ancillary testing based on exam findings may be needed, such as optical coherence tomography.
She added, “I would like to stress that most of the ophthalmic adverse effects can be managed locally, with continuation of immunotherapy in select cases. However, if these ocular problems are not caught early and treated appropriately, they can be visually devastating.”
—Linda Roach
___________________________
1 Kim JM et al. Ophthalmology. Published online Feb. 5, 2019.
___________________________
Relevant financial disclosures—Dr. Pointdujour-Lim: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Bharti None.
Dr. Koch Alcon: C; Carl Zeiss Meditec: C; Johnson & Johnson: C.
Dr. Nouri-Mahdavi Heidelberg Engineering: L,S.
Dr. Pointdujour-Lim None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review