Download PDF
Ophthalmological applications of robot-assisted surgery essentially are in their teenage years, still proving their identity and what they can accomplish. First evaluated for cataract surgery, these technologies have moved into vitreoretinal surgeries, with the first-in-human studies and first approved product appearing in Europe.
Inherent Challenges
This new world of surgery poses myriad challenges for those who are developing robotics platforms. In vitreoretinal surgery, for instance, the surgeon manipulates tissues that can be thinner than a human hair, at forces below what he or she can actually feel. Surgeons contend with inadequate spatial resolution and depth perception of microstructures, natural hand tremor, and difficulty feeling the force to use, for instance, to peel a membrane.1
It is these challenges that researchers are seeking to address with robotic technologies. Here’s a look at three contenders in ophthalmology—and an overview of potential applications.
Option One: Steady Hand
One strategic approach to compensate for the human limitations includes creating feedback loops for a surgeon’s visual and tactile senses.
Enter researchers at Johns Hopkins University in Baltimore. Their hand-held robotic system, the Steady Hand, is a cooperative-assist robot. “The surgeon grasps the robotic tool, and the robot and the human work cooperatively,” said Peter L. Gehlbach, MD, PhD, at the Wilmer Eye Institute and the Whiting School of Engineering at Johns Hopkins.
Designed around an XYZ axis to move tools in any direction, this robot has a roll mechanism that moves the arm for better eye access and a tilt mechanism to change the angle of an instrument. “The robot conveys the usual advantage of eliminating tremor,” said Dr. Gehlbach. “It’s very precise but has the usual limitations of restricting the degrees of motion.”
Microforce and distance sensors. Managing intraoperative microforce relationships is critical to successful surgical outcomes. “Where the forceps or scissors touches the retina, if you ask surgeons to close their eyes, they can’t tell if they’re touching or not in traditional surgery,” Dr. Gehlbach said. “Presently, vitreoretinal surgery remains a 99% visual task where the tool tip meets the retina.”
Smart instruments have already advanced ocular surgery beyond the limits of human visual and tactile sensing, so robot technology seeks to surpass that horizon. The Hopkins team is developing microforce sensors to integrate into its robotic technology. “The total forces applied to the retina are so minute that humans can’t generally feel them,” said Dr. Gehlbach. “But robotic tools can be designed to ‘feel’ very small forces and provide feedback to a surgeon not otherwise available.”
They have also developed distance sensors for the robot tool tip. “A robot could know how to barely touch tissue and how to avoid it,” Dr. Gehlbach said, “and into that controlled feedback loop, you can begin to incorporate artificial intelligence–guided tasks so robots can learn to do new things.”
Force generation and hand tremor. To solve this problem, the Steady Hand robot measures and reports force generation. “If the tool tip grasps the retina and you pull on it, you can see the resulting ‘force signature’ for whatever you’re doing,” said Dr. Gehlbach. “What if an artificial intelligence application understood what that force signature meant—where the danger zones and safe zones were—and could send an ‘informed’ assist to the surgeon for safety and efficacy?”
Robot technology can surpass human limitations in multiple ways. Retina surgeons have a natural hand tremor with an average amplitude of 156 μm.2 “A human can’t hold the tip of a tool steady inside a blood microvessel for several minutes, but a robot can,” said Dr. Gehlbach. “A robot enables a surgeon to potentially improve safety and efficacy, and it opens a vista for potential new procedures.”
Current status. The Steady Hand is still in preclinical development. “It’s scientifically challenging, fascinating from an intellectual point of view, potentially entrepreneurial, with a lot of creative energy going into it,” Dr. Gehlbach said.
 |
|
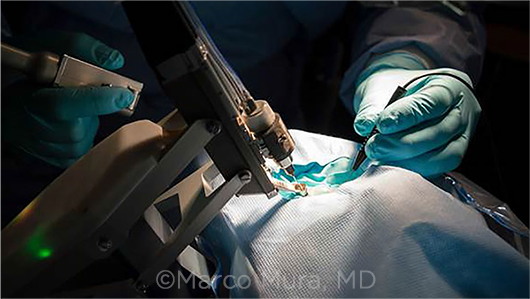
ON THE MARKET. The Preceyes platform was the first robot-assisted surgery system to be used in human eyes. It recently received the European CE mark, making it eligible for use outside of clinical trials there.
|
 |
|
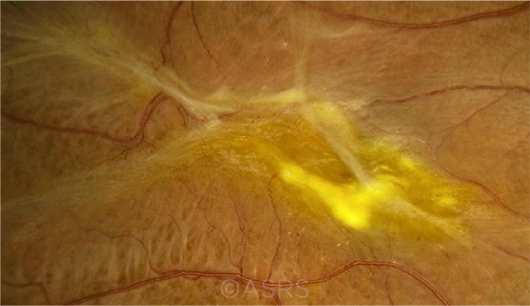
RESEARCH TARGET. Researchers used the Preceyes platform for peeling the epiretinal membrane (shown here) or the inner limiting membrane. This image was originally published in the ASRS Retina Image Bank. Sharon Fekrat, MD, FACS. Epiretinal Membrane. Retina Image Bank. 2012; Image Number 1437. © The American Society of Retina Specialists.
|
Option Two: Preceyes
Originating in the Netherlands, the Preceyes Surgical System (Preceyes BV) is currently in use in England, the Netherlands, and Switzerland.
Setup. This system positions the surgeon at the head of an operating table with the robot attached to a headrest.3 “Its hybrid design allows the surgeon to decide when and how to use the robot during a procedure,” said Marco Mura, MD, at King Khaled Eye Specialist Hospital in Riyadh, Saudi Arabia. “A motion controller positioned in the surgical field records the surgeon’s movements, which are filtered and enhanced by a computer prior to being fed to a tissue manipulator, all of this in real time,” he said.
The Preceyes platform is approved in Europe to perform vitreoretinal procedures such as macular surgery, retinal membrane peeling, supraretinal injections, and intravenous cannulation, said Dr. Mura, also at the University of Illinois at Chicago. “As a next step, we’re now evaluating optical coherence tomography (OCT) distance-sensing at the instrument’s tip to increase precision and safety of intraocular tasks.”
“In our initial studies on precision, we showed that the device is at least 10 times more precise than a human hand,” Dr. Mura said. “Such precision will provide us with better clinical outcomes in a less stressful environment.”
First-in-human study. Surgeons from the Nuffield Laboratory of Ophthalmology in Oxford, England, published the first-in-human study of the safety and viability of intraocular robot-assisted surgery. For the study, 12 patients were randomly assigned to traditional or robot-assisted macular surgery using Preceyes. All patients required either removal of the epiretinal membrane (ERM) or inner limiting membrane (ILM), and the study focused on the most demanding surgical step: peeling the flap of ERM or ILM away from the macular surface.3
The results? “Safety was demonstrated both in the peeling task using general anesthesia and in a subretinal injection task using local anesthesia,” said Dr. Mura. “Fewer inadvertent retinal touches and resulting retinal microtrauma were observed in the robot cases than in the controls; and although this did not reach statistical significance, the absence of any obvious difference was supportive of the robotic system’s safety profile,” he said.
Dr. Mura also noted that “speed was always sacrificed in the interest of safety, which biased the surgery duration in favor of the standard manual technique, for a median time over four minutes versus over one minute.”3
Precision finer than a human hair. A human hair measures 50 μm in diameter. The ERM has a mean thickness of 61 μm, while the ILM is less than 20 μm. The Preceyes system is designed with a protective “virtual Z boundary” function that controls the robot along its Z axis, perpendicular to the retina. This allows for a tool’s incremental steps forward until touching down on the membrane itself. The system’s software includes functions that store a tool’s position in the eye, variably scale instrument motion, return the tool to previous positions, and pause in stand-by mode.3
With both visual and intraoperative OCT guidance, the surgeon can move an instrument to within 100 μm of the retina and advance with small nudges of 10 to 50 μm into the tissue plane.3 Surgeons and engineers write each step in a procedure’s software so the robot can direct the speed and depth of instrument movements.
“In the future, we will probably be able to automate critical surgical steps,” Dr. Mura said, “but at the moment, the robot is just assisting, enabling surgeons to execute tasks that require a stability and reproducibility that are out of reach for manual surgery, and improving precision and reliability.”
Use for gene therapies? The Nuffield study also randomized six patients into manual or robot-assisted injection of rtPA (recombinant tissue plasminogen activator) to displace submacular hemorrhage and reported successful procedures for all six. As a potential safety-related benefit, the researchers noted the robot can pause to allow for a slow, drip-like subretinal infusion instead of a faster manual injection.3
“All the cell-based therapies, like stem-cell and gene therapy, require very precise injection of these cells,” Dr. Mura said, “so robotic surgery is opening an exciting new chapter in ophthalmology.”
Current status. Now that the Preceyes platform has received the European CE mark, “We can have it purchased by hospitals and used in clinical practice,” Dr. Mura said.
 |
|
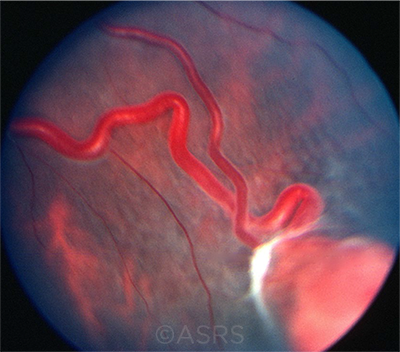
CHEMO DELIVERY? One potential application for robot-assisted surgery: delivering medication to intraocular tumors via feeder vessels (shown here). This image was originally published in the ASRS Retina Image Bank. From the Collections of Thomas M. Aaberg, MD, and Thomas M. Aaberg Jr., MD. Retinal Hemangioblastoma. Retina Image Bank. 2013; Image Number 4687. © The American Society of Retina Specialists.
|
 |
|
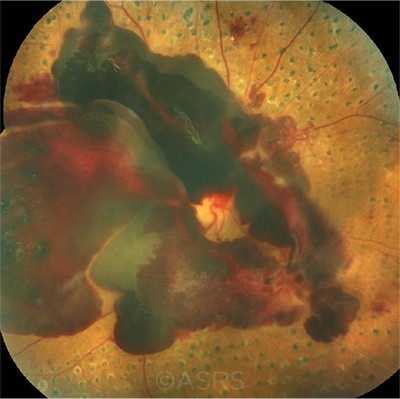
IN WHOSE HANDS? Will robotic systems eventually be used to administer injections for hemorrhages such as this one? This image was originally published in the ASRS Retina Image Bank. Awaneesh M. Upadhyay, MBBS, DNB, Dr. Diakant Misra, Dr. PushKar Dhir, and Dr. Ronel Soibam. Subhyaloid Hemorrhage, Proliferative Diabetic Retinopathy. Retina Image Bank. 2018; Image Number 28252. © The American Society of Retina Specialists.
|
Option Three: The UCLA Platform
At the University of California, Los Angeles (UCLA), researchers are working on the Intraocular Robotic Interventional Surgical System. “We have two main goals,” said Jean-Pierre Hubschman, MD, at UCLA’s Stein Eye Institute. “We’re working to develop a fully automated cataract surgical robotic platform—and to develop a vitreoretinal robotic platform using augmented feedback, both visual and tactile, to assist the surgeon in performing retinal surgery.”
A “master/slave” design. The UCLA platform positions the robot between patient and surgeon. This approach, called a “master/slave” design, involves the surgeon working from a surgical cockpit.
“The surgeon’s input is filtered and processed by the robotic technology,” said Dr. Hubschman. “Surgical instruments are controlled through the robotic platform, which integrates a real-time OCT loop, so it will limit the surgeon from pulling the retina too much or entering a forbidden space.”
Human reaction times are considerably slower than data processing speeds. “If you see the patient moving, it takes 300 milliseconds for a human to react,” said Dr. Hubschman. “A robotic system takes only a few milliseconds.”
Current status. As with other U.S. platforms, the system is not yet ready for clinical trials. “For cataract surgery, we tested our platform on 30 harvested pig eyes and achieved complete lens removal in all cases without any capsular rupture or damage, but we need another three to four years to finalize the prototype to be tested on humans,” said Dr. Hubschman.
Steven D. Schwartz, MD, who recruited Dr. Hubschman to UCLA to lead projects such as the robotics initiative, predicted that in five years, initial clinical tests for robotic cataract surgery will be complete, with tests for retina surgery occurring several years after that. This extended research and development timeline is one benefit of UCLA’s support, he said, as “large academic centers like UCLA can do things that even industry might not do to move the field forward.”
A note on collaboration and automation. The UCLA project draws inspiration and resources from multiple entities, including the Stein Eye Institute, the Center for Advanced Surgical and Interventional Technology at UCLA, and the NEI.
And Dr. Schwartz emphasized just how critical this collaboration is. “We’re extremely fortunate to have spectacular groups involved: engineers who worked on the Hubble Space Telescope, physicists who worked on the Hadron Collider, and computer scientists and bioengineers who’ve built exoskeletons for the military,” he said.
Another element central to the UCLA project is automation, Dr. Schwartz said. “By using a combination of data input from intraocular OCT, extraocular OCT, ultrasound, and ultrasound biomicroscopy, there’s a huge amount of data going into a signal processing unit. We’re combining these incredible technologies into one system that will fully automate surgery in the future.”
Nonetheless, Dr. Schwartz said, “this technology is not intended to replace the surgeon; it’s intended to improve care and deliver personal, compassionate care. The idea of the robot is to help us in the quest to improve.”
___________________________
1 Nuzzi R, Brusasco L. Eye Brain. 2018;10:13-24.
2 Channa R et al. Retina. 2017;37(7):1220-1228.
3 Edwards TL et al. Nat Biomed Eng. 2018;2:649-656
A Brave New World
What more might the future hold for robotic surgery in ophthalmology?
Medical education. “One of the most powerful uses of robot-assisted surgery may be in surgical education,” said James T. Handa, MD, at the Wilmer Eye Institute in Baltimore. “Its capabilities are greatest in objectifying and quantifying every surgical movement to develop a language of surgery.”
As Dr. Handa pointed out, surgical students may be too aggressive or timid. “They remove the membrane so fast that they pull the retina off, or they pull at one-tenth of what’s needed,” he said. “But if you can give them physiological feedback on what they’re doing, their performance improves rapidly.”
Robot technology also improves safety in surgical education. “The robot builds in ‘safe ways’ so you don’t exceed certain parameters,” Dr. Handa said. And the technology could potentially be used to reach more learners, evolving from one-to-one to one-to-many students.
Other applications. “Other uses of the technology may lie in standardizing surgical technique during clinical trials, evaluating surgeon competence in medicolegal cases, and delivering chemotherapeutics into feeder vessels of intraocular tumors to improve efficacy and reduce toxicity,” Dr. Handa said.1
Theoretically, the master/slave model could be extended over miles, even continents, for remote surgery. “Imagine a world where an expert surgeon sits in the United States, and a military trauma doctor in a field hospital could be guided remotely from two continents away,” said Dr. Schwartz.
Remaining barriers. A number of barriers still need to be overcome before robot technology reaches its full surgical potential in the United States, including surgeon and patient acceptance and such practical issues as operating time, initial cost outlays, technical maintenance, and insurance coverage.2
But if these challenges are met, is there any part of surgery that won’t eventually be taken over from the surgeon’s hands or eyes? Dr. Gehlbach responded, “The collection of the paycheck, I’m afraid. You couldn’t wrestle that from human hands.”
___________________________
1 Channa R et al. Retina. 2017;37(7):1220-1228.
2 Nuzzi R, Brusasco L. Eye Brain. 2018;10:13-24.
|
Meet the Experts
Peter L. Gehlbach, MD, PhD Professor of ophthalmology at the Wilmer Eye Institute and on the faculty at the Whiting School of Engineering at Johns Hopkins in Baltimore. Relevant financial disclosures: None.
James T. Handa, MD Robert Bond Welch Professor of Ophthalmology and chief of the Retina Division at the Wilmer Eye Institute in Baltimore. Relevant financial disclosures: None.
Jean-Pierre Hubschman, MD Associate professor of ophthalmology and director of the Advanced Robotic Eye Surgery Laboratory at UCLA’s Stein Eye Institute in Los Angeles. Relevant financial disclosures: Alcon: C; Allergan: L; Bausch + Lomb: C; NEI: S; Novartis: C.
Marco Mura, MD Visiting professor at the University of Illinois at Chicago and chief of the Retina Division at King Khaled Eye Specialist Hospital in Riyadh, Saudi Arabia. Relevant financial disclosures: Preceyes: C.
Steven D. Schwartz, MD Ahmanson Professor in Ophthalmology and chief of the Retina Division at UCLA’s Stein Eye Institute in Los Angeles. Relevant financial disclosures: California Institute for Regenerative Medicine: S; NEI: C,S; Verana Health: O.
Full Financial Disclosures
Dr. Gehlbach Johns Hopkins University: P; NIH: S.
Dr. Handa Bayer Pharmaceuticals: S; BrightFocus Foundation: S; Foundation Fighting Blindness: S; Macular Degeneration Foundation: S; NEI: S.
Dr. Hubschman Alcon: C; Allergan: L; Bausch + Lomb: C; NEI: S; Novartis: L.
Dr. Mura Alcon: C; Allergan: C; Bayer Healthcare: C; Preceyes: C.
Dr. Schwartz California Institute for Regenerative Medicine: S; NEI: C,S; Verana Health: O.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|