Download PDF
With the 25th anniversary of the groundbreaking Studies of Ocular Complications of AIDS, four experts reflect on the achievements of the past—and the challenges of the future—in treating HIV-related eye disease.
The acquired immunodeficiency syndrome (AIDS) epidemic has had a profound impact on ophthalmology. Ocular complications of infection with the human immunodeficiency virus (HIV) were first described in 1982 by Gary N. Holland, MD, at the University of California, Los Angeles.1 Seven years later, the Studies of Ocular Complications of AIDS (SOCA) was initiated as a multicenter research effort funded by the National Eye Institute. No one could have predicted how the AIDS epidemic and its effects on the eye would be transformed over the next 25 years. “The value of SOCA is that its focus changed as the AIDS epidemic evolved," said Dr. Holland.
"Because we're no longer seeing devastating vision loss very frequently in the United States, many ophthalmologists have the feeling that people living with HIV are no longer at high risk for ocular complications,” said Dr. Holland, “but there are also chronic changes occurring in the eye that can affect a patient's quality of life, and those changes can occur despite immune recovery. Many of the things going on systemically in an HIV positive person can affect the eye as well."
Since 1998, the Longitudinal Study of Ocular Complications of AIDS (LSOCA)—a sub study within SOCA—has been examining the risk of ocular complications over time and the effects of long-term treatment on visual function, quality of life, and survival. Understanding the ocular changes associated with HIV may have important implications for other eye diseases, such as macular degeneration, as well as many systemic diseases.2,3 Here is an update on key research findings for clinicians.
 |
|
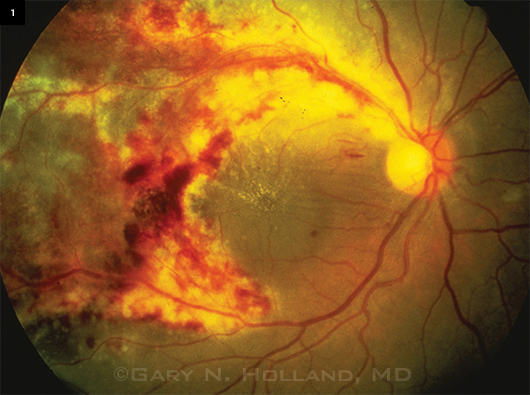
Untreated CMV. The appearance of untreated cytomegalovirus (CMV) retinitis in a severely immunodeficient patient, early in the AIDS epidemic, before the availability of anti-CMV drugs or antiretroviral drugs.
|
Before HAART: Blinding infections
“The biggest problem we had when SOCA was first funded was opportunistic ocular infections,” said SOCA chairman Douglas A. Jabs, MD, MBA, at the Icahn School of Medicine at Mount Sinai in New York City. “Cytomegalovirus [CMV] retinitis accounted for about 90 percent of the AIDS-related eye infections. By the early 1990s, it had become the most common intraocular infection in urban centers.”4
CMV retinitis. About a third of AIDS patients contracted CMV retinitis (Fig. 1), which progressively destroys retinal tissue, causing irreversible vision loss. “AIDS patients feared blindness more than anything else,” said David Heiden, MD, at Pacific Eye Associates in San Francisco. “Many knew they were going to die and just didn’t want to go blind first.” CMV-associated diseases are late-stage opportunistic infections that make people sick only when their immune system crumbles significantly (CD4+ T cells less than 50 cells/µL). In the 1980s, when patients got CMV retinitis, their life expectancy was about three months, according to Dr. Heiden.
The introduction of highly active antiretroviral therapy (HAART) in 1996 was a watershed event in the history of the AIDS epidemic—“a revelation,” said Dr. Heiden. “Immune recovery became a reality, and new cases of CMV retinitis largely disappeared over the next year or two.”
 |
|
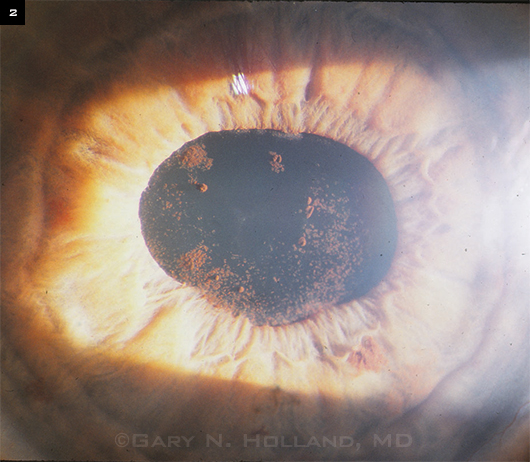
IRU Effects. Anterior segment of a patient with IRU in 1997, soon after the introduction of HAART. The patient had a history of CMV retinitis and was treated with the anti-CMV agent cidofovir, a drug later shown to be a risk factor for IRU. Improvement in immune function as a result of antiretroviral therapy led to an inflammatory response against CMV antigens in the eye, causing anterior uveitis. Pigment clumps seen on the anterior lens capsule are remnants of posterior synechiae that were successfully broken with dilation.
|
The HAART Transition
Immune recovery induced by HAART brought profound benefits—but also new difficulties—to people with AIDS-related CMV retinitis.
Immune recovery uveitis. With immune recovery, patients’ own immune defenses were able to suppress CMV, allowing CMV retinitis lesions to become inactive without the need for antiviral drugs. However, as a consequence, a new condition emerged among treated patients: immune recovery uveitis (IRU). With immune reconstitution, some patients develop heightened immunological reactions against CMV or other intraocular pathogens, resulting in ocular inflammation. The inflammation is most prominent in the vitreous cavity but can also involve the anterior segment (Fig. 2). IRU may become severe enough to cause hypopyon and lead to vision-threatening complications such as synechiae formation, inflammatory membranes, cataract, and macular edema.
Evolving treatment strategies. These developments necessitated a new approach to the management of CMV retinitis. “When HAART became available, we needed appropriate strategies for controlling infectious retinopathies while preventing the inflammatory damage that comes with IRU. Our focus was getting patients through the period of immune recovery without added ocular complications secondary to IRU,” said Jennifer E. Thorne, MD, PhD, deputy director of the Coordinating Center of SOCA.
“We were adapting the treatments we used when life expectancy was short to a more prolonged period of CMV disease,” Dr. Holland said. “We had to figure out when it was safe to discontinue anti-CMV drug treatment in patients who achieved immune recovery.” In general, anti-CMV therapy should be maintained until the retinitis is inactive and CD4+ T cell counts greater than 100 to 150 cells/µL have been documented for at least six months.5
Ongoing vigilance needed. Although AIDS has converted to a chronic disease, long-term monitoring for CMV remains important. CMV reactivation is possible (albeit at a much lower rate), even if immune recovery is maintained; and complications from prior CMV retinitis, such as retinal detachment, may require treatment.3 Further, despite the widespread availability of HAART, clinicians should be aware that, though infrequent, new cases of CMV retinitis may occur.
CMV Retinitis: Disease Is Down, but Far From Out
The incidence of CMV retinitis declined in the United States by more than 95 percent after HAART was introduced, according to Dr. Jabs. But the incidence is not zero. “The leading cause of visual loss in people with AIDS is still CMV retinitis,” he said.
According to Dr. Holland, the increasing longevity of HIV-infected people means that the cumulative numbers of patients with the sequelae of infectious retinopathies will likely grow. In addition, it is feared that as HIV resistance to antiretroviral drugs increases, there may also be a rise in new cases of CMV retinitis.
“SOCA’s recent data suggest that, despite HAART, developing CMV retinitis is a risk factor for AIDS-related death,” said Dr. Thorne. Early diagnosis is the best way to reduce the complications of CMV, making regular screening in HIV-positive patients essential. (See “Advice for Comprehensive Ophthalmologists.”)
“SOCA was incredibly valuable in describing treatment outcomes for CMV retinitis in the earliest parts of its history,” said Dr. Thorne. Its legacy is that we have very well established treatment guidelines and regimens to control CMV, she explained.
“Because we have so much experience treating CMV retinitis in AIDS patients, the means to treat CMV infection have become available to other groups that develop viral infections to the retina, such as newborns or immunocompromised patients, including organ transplant recipients,” said Dr. Holland.
The same is true for the emerging epidemic of CMV retinitis in developing countries. “CMV retinitis is the neglected disease of the AIDS pandemic in the developing world,” said Dr. Heiden. “People in resource-limited areas need the type of eye care that we learned to do very well in the pre-HAART era. We need to extend that care to them.”
Watch for next month’s Clinical Update focusing on international aspects of HIV eye disease. |
Current Focus: Chronic Conditions
“If you look back at the medical issues related to managing the AIDS epidemic, it went from being primarily concerned with treating opportunistic infections to managing immune recovery and immune recovery complications to managing a chronic disease and now to managing age-related diseases in HAART-treated, immunorestored patients,” said Dr. Jabs.
Age-related diseases. Compared with individuals without HIV infection, said Dr. Jabs, these patients “have more cardiovascular disease, diabetes, osteoporosis, and dyslipidemia, at a younger age. They have immune systems that look like people much older than they are.”
“There also is a concern that there may be a greater rate of age-related ocular changes, such as cataract, at a younger age than we might expect in the HIV-uninfected population,” said Dr. Thorne. “It’s not completely clear yet whether there are more age-related eye diseases,” she said, “but it’s definitely SOCA’s area of current interest.” Dr. Jabs added, “We hope to learn more about the question of accelerated aging in the eye of HIV-infected persons over the next five years of LSOCA.”
Microvasculopathy: ocular and systemic. Even without infectious retinopathies, HIV-infected patients may have subtle visual disturbances with functional consequences, such as visual field loss and abnormalities in contrast sensitivity and color vision. Although the prevalence of these problems is relatively low, they are still three or four times more common than in the general population. They are thought to be caused by abnormalities of the retinal vasculature at the microscopic level, which may be analogous to the systemic microvasculopathy known to occur in HIV disease.3
“We know that some of the presumed contributors to HIV-associated microvasculopathy—such as activation of white blood cells and abnormal blood flow—are ongoing despite HAART, and we anticipate that patients may, therefore, develop more eye problems in the future,” said Dr. Holland.
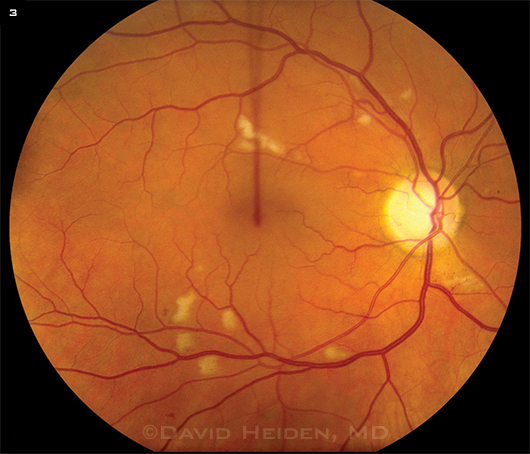
“In medical school, you always hear that what’s going on in the eye is a reflection of what’s going on elsewhere in the body,” he continued. Retinal vascular damage (Fig. 3) seems to be related to systemic vascular damage and does correlate with mortality.6
“Retinal microvasculopathy could serve as a marker to help establish the severity of vessel-associated diseases throughout the body,” said Dr. Holland. “It may be like diabetic retinopathy, where we know that similar microvascular changes are occurring systemically. With many years of living with HIV, is the retinal damage going to progress the way it does with diabetic retinopathy? If so, are there implications for understanding HIV disease in other organs?” These questions, too, are under investigation in LSOCA.
 |
|
Retinopathy. Fundus of patient with HIV retinopathy shows characteristic cotton-wool spots.
|
Advice for Comprehensive Ophthalmologists
Most of the risk to the eye in people living with HIV relates directly to their level of immune deficiency. “For anyone who is immunorestored and has no AIDS-related ocular problems, a comprehensive ophthalmologist probably can follow that patient as well as any specialist,” said Dr. Jabs.
The typical HIV-positive patient seen in a general ophthalmology practice is on a stable antiretroviral regimen, has good laboratory parameters, and has no history of eye disease. Once-a-year routine care is fine for these patients, according to all the physicians interviewed for this article. Following are some considerations for clinicians caring for people with HIV.
Be aware that CMV may still occur. CMV retinitis remains the most frequent ocular opportunistic infection, although it occurs almost exclusively in people with CD4+ T cell counts of less than 50 cells/µL; and even among these patients, the risk has decreased from the pre-HAART era.7 The risks of non-CMV infections (e.g., herpetic retinitis, toxoplasmic retinitis, and fungal or mycobacterial choroiditis) in the HAART era are very low,8 but for those who do get them, the results can be devastating, Dr. Jabs said.
Today, most patients presenting with active CMV retinitis fall into three categories: 1) people infected with HIV who have not yet been diagnosed and are HAART naive; 2) patients who’ve become resistant to their HAART or are intolerant of the regimen; and 3) patients who are nonadherent to their prescribed therapy.2
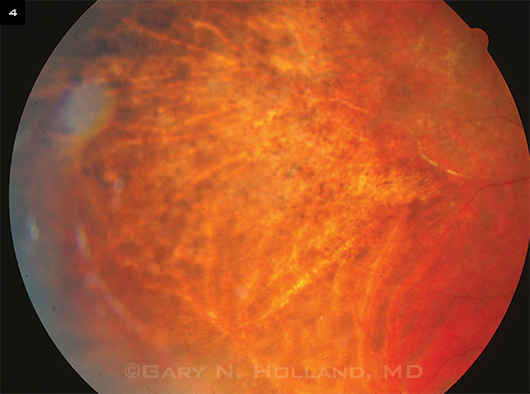
Screen for HIV complications. Screening patients for CMV retinitis and other complications of HIV requires a dilated exam to evaluate the entire retina, as CMV retinitis often presents as a peripheral retinitis (Fig. 4). “Patients who develop CMV retinitis with lesions in the peripheral retina may have no ocular symptoms to alert them to an eye problem,”9 said Dr. Thorne. Evidence of immune suppression in lab results (low CD4+ T cell count and high HIV load) should raise red flags for the ophthalmologist. “We can’t depend on symptoms alone,” she cautioned.
Watch for long-term sequelae. Rather than seeing new cases, ophthalmologists are more likely to encounter long-term survivors who have inactive CMV retinitis but who may still have visual problems caused by the previously active infection. Challenges in this group include IRU, cataracts, possible reactivation (even with good immune function, in rare cases), risk of retinal detachment, and presence of silicone oil from prior retinal repair. Some patients will benefit from interventions such as cataract surgery or removal of silicone oil from the eye.10
When to refer. “Should a patient develop a severe HIV-related problem, the general ophthalmologist may be more comfortable referring the patient to someone with experience managing that particular eye disease,” said Dr. Holland. “This is particularly true for the new generation of ophthalmologists, many of whom may never have seen a case of CMV retinitis during their training.”
Follow-up schedules. Some patients require more frequent follow-up. “Patients who’ve had eye problems in the past, like CMV retinitis or IRU, I see more frequently,” said Dr. Holland. Dr. Thorne sees her HIV-positive patients with a CD4+ T cell count over 200 cells/µL annually; a CD4+ T cell count between 50 and 200 cells/µL, every six months; and a CD4+ T cell count under 50 cells/µL, every three months.
Dr. Holland added, “It’s very important for patients to be educated about what signs and symptoms should alert them to call their ophthalmologist immediately. If their CD4 count falls or they have viremia and/or eye symptoms—any changes in vision, but especially floaters, blind spots, and blurriness—they need to know to come in right away for an evaluation.”
 |
|
Peripheral Findings. The peripheral fundus of an eye with CMV retinitis. This patient was being treated with the anti-CMV drug ganciclovir but had not yet started HAART. The patient later began antiretroviral therapy and experienced immune recovery, after which ganciclovir treatment was stopped. No subsequent reactivation has occurred at the border of the scarred region.
|
Looking Back, Moving Forward
“Though we’re learning more from LSOCA about what to expect in long-term survivors, I tell my patients we’re stepping into the unknown,” said Dr. Heiden, to encourage his patients to return regularly for evaluation.
In summary, Dr. Jabs said, “If you look back at the AIDS epidemic, we went from being primarily concerned with opportunistic infections to managing immune recovery and immune recovery complications to managing a chronic disease and now to managing age-related diseases. SOCA has enhanced our understanding of the eye problems that people living with HIV and AIDS are going to have and how to manage them. The AIDS epidemic will continue to evolve, and we want to be able to tell patients what to expect and how to plan ahead.”
__________________________
1 Holland GN et al. Am J Ophthalmol. 1982;93(4):393-402.
2 Studies of Ocular Complications of AIDS (SOCA): Curriculum Vitae. January 2012. Prepared by SOCA Coordinating Center, Baltimore. Accessed Oct. 23, 2013.
3 Holland GN. Am J Ophthalmol. 2008;145(3):397-408.
4 McCannel CA et al. Am J Ophthalmol. 1996;121(1):35-46.
5 Guidelines for Preventing Opportunistic Infections Among HIV-Infected Persons, 2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Accessed November 27, 2013.
6 Holland GN et al. Am J Ophthalmol. 2010;149(5):807-816.
7 Sugar EA et al. Am J Ophthalmol. 2012;153(6):1016-1024.e5.
8 Gangaputra S et al. Am J Ophthalmol. 2013;155(2):206-212.e5.
9 Jeng BH et al. Surv Ophthalmol. 2007;52(4):329-368.
10 Holland GN et al. Am J Ophthalmol. 2008;145(1):12-22.
Meet the Experts
David Heiden, MD Uveitis consultant and general ophthalmologist at Pacific Eye Associates, San Francisco; director of international ophthalmology, California Pacific Medical Center; and medical director, AIDS Eye Initiative, Seva Foundation. Financial disclosure: None.
Gary N. Holland, MD Jack H. Skirball Professor of Ocular Inflammatory Diseases, David Geffen School of Medicine at University of California, Los Angeles; and a member of the SOCA Steering Committee. Financial disclosure: Is a member of the medical advisory board of Novartis and XOMA.
Douglas A. Jabs, MD, MBA Professor and chair, department of ophthalmology and professor of medicine, at Icahn School of Medicine at Mount Sinai, the Mount Sinai Health System, and the New York Eye and Infirmary, New York; and chairman of SOCA. Financial disclosure: Has consulted for Abbott, Alcon, Allergan, Corcept Therapeutics, Genentech, Genzyme, GlaxoSmithKline, and Roche, and serves on the data and safety monitoring committees for Applied Genetic Technologies and Novartis.
Jennifer E. Thorne, MD, PhD Professor of ophthalmology and epidemiology and the director of the Division of Ocular Immunology at the Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore; and deputy director of the Coordinating Center of SOCA. Financial disclosure: Receives research funding from the NEI, NIAID, Research to Prevent Blindness, and Allergan; consults for AbbVie, Gilead, and XOMA. |