Download PDF
When it comes to treating dry eye disease (DED), some ophthalmologists may be skeptical of the hype surrounding the latest drugs and devices to hit the market. They caution that until there is a better understanding of DED’s underlying mechanisms, therapeutic and diagnostic breakthroughs may remain elusive.
Still, ophthalmologists with an interest in dry eye have reasons for optimism. Dry eye experts are noting incremental advances in diagnosis and treatment in their daily practices. “From my perspective, there have been major improvements already,” said Elisabeth M. Messmer, MD, at Ludwig-Maximilian University of Munich in Germany.
Update on Therapies
Innovations. According to Dr. Messmer, today’s artificial tears include ingredients that enable them to remain longer on the ocular surface. These include compatible solutes (osmoprotectants), lipids, and molecules that work as secretagogues. Even more important, artificial tears without the toxic preservative benzalkonium chloride are now readily available, she said.
Penny Asbell, MD, at the University of Tennessee Health Science Center in Memphis, reported that new molecules to replace missing elements in the tears, such as proteoglycans, and novel antioxidative drugs, such as SkQ1, are coming down the pike. In particular, Giacomina Massaro-Giordano, MD, at the University of Pennsylvania in Philadelphia, is excited about a glycoprotein lubricant called lubricin, which is different from carboxymethylcellulose and all previous lubricants. She is also optimistic about a synthetic form of lacritin—a protein that is selectively deficient in dry eye tears and stimulates tear secretion and corneal epithelial renewal. In addition, nerve growth factor drops (cenegermin, Oxervate), recently FDA approved for neurotrophic keratitis, may help a subset of dry eye patients.
Anti-inflammatories. Anti-inflammatory drugs on the market include cyclosporine A (Restasis, CSA 0.05% in the United States; Ikervis, 0.1% in Europe) and lifitegrast (Xiidra). There is a role for these medications, though the benefits depend on careful patient selection, according to Sonal S. Tuli, MD, MEd, at the University of Florida in Gainesville. For starters, most patients with inflamed eyes cannot tolerate the drops. “To make cyclosporine into an emulsion, you have to make it a little acidic,” Dr. Tuli explained. “It can feel like putting lemon juice on a wound.” To help patients tolerate Restasis or Xiidra, most clinicians first prescribe steroids to calm the eye, according to Joanne F. Shen, MD, at Mayo Clinic in Arizona, who is not a fan of the so-called Lotemax-to-Restasis bridge. “Once patients start on steroids, they don’t want to come off. Many patients will need cataract surgery earlier than would have been expected [because the steroid can cause cataract formation],” she cautioned.
Dr. Tuli sometimes opts to bridge a patient to Restasis with a milder steroid for a week, but she insists on a strict, rapid taper. Both Drs. Tuli and Shen are proponents of doxycycline and often choose to skip the Restasis- or Xiidra-plus-steroid approach.
Newer formulations of cyclosporine (e.g., the combination of cyclosporine with semifluorinated alkanes to improve bioavailability) are in the study pipeline, according to Drs. Messmer and Asbell; results are expected soon. One new nanotechnology formulation, a nanomicellar formulation of cyclosporine A (0.09%) called Cequa was FDA approved in 2018 but is not yet commercially available. Generic versions of CSA were expected to enter the U.S. market last year but have been delayed, said Dr. Tuli.
Devices. Mechanical options are also available to treat meibomian gland dysfunction (MGD), including LipiFlow and intense pulsed light (IPL). An intranasal neurostimulator, TrueTear, is now sold over the counter. Despite the hefty price tag for the latter, Dr. Tuli said that some patients really like it. However, why or how long it will continue to have a sustained effect after its use is discontinued is still unknown, she said.
Diagnostic Considerations
Diagnostic tools. Today, diagnostic tools to detect inflammation (e.g., biomarkers like MMP-9) and corneal innervation are recognized as important for individualizing treatment. Dr. Asbell is most excited about the potential for minimally invasive objective metrics. “The development of well-validated biomarkers would allow us to more specifically categorize DED problems and tailor our treatments accordingly,” she said.
Diagnosis is paramount. Often, the takeaway from expert discussions about DED is to spend time performing an exhaustive examination to pin down the cause of dry eye. Therapies can differ completely, depending on whether the etiology lies in the tear film, anatomy, or nervous system—or in a combination of these. Moreover, the cause can have an impact on how aggressive your approach might be. For example, when Dr. Tuli sees patients with Sjögren syndrome, a progressive autoimmune process that will continue to damage their tear glands, she starts them on one of the prescription drugs right away, even if the patient doesn’t have symptoms. But she wouldn’t be so aggressive for a patient with dry eye induced by computer use.
Make the time for workup. According to Dr. Shen, it’s extremely difficult in most practices to find the time needed to rule out everything that could be masquerading as dry eye or contributing to it. Dr. Massaro-Giordano agreed and emphasized the importance of conjunctival staining, especially with lissamine green. Dr. Messmer added, “With a drop of fluorescein, you can judge three important things at the same time to establish a dry eye diagnosis: 1) the tear film meniscus, 2) tear film break-up time [TBUT], and 3) ocular surface damage.”
Make time for patient interaction. The experts advise ophthalmologists to set aside adequate time for dry eye patients. “Show your patients some love,” said Dr. Asbell. “Listen to them carefully and see them regularly if they’re very symptomatic.” Dr. Messmer added, “Take your patients’ complaints seriously. Some may complain of visual disturbances although they have full vision on conventional vision testing. This is due to a decrease in functional visual acuity.”
Looking Ahead
Some ophthalmologists have developed a healthy level of skepticism about new products. But, said Dr. Massaro-Giordano, given the vast number of DED patients and the many therapies in development to address different mechanisms and symptoms, it’s important for ophthalmologists to keep a keen eye on the pipeline and an open mind.
“It’s hard to say why some things work for dry eye,” said Dr. Massaro-Giordano, “They just do. Over 25 years, I’ve seen it clinically, though sometimes it’s hard to pinpoint the science behind it.” For those waiting for evidence on whys and hows of various treatments, she said, “The science is coming!”
Case Studies
The cases on the next few pages help elucidate the diagnostic process and therapeutic approaches—often combining newer therapies with the trusted standbys—followed by experts in the field. The key messages are recognizing that DED is a multifactorial process, tailoring treatment to the particulars of the individual case, and being prepared to escalate to more intensive therapy when response is inadequate.
CASE 1: Neuropathic Ocular Pain, or “Pain Without Stain”
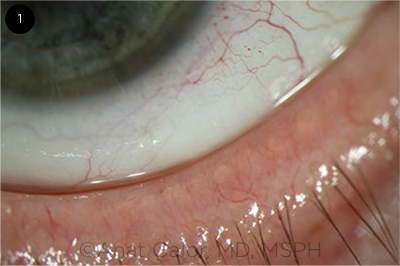 |
|
CASE 1. Mild meibomian gland dysfunction.
|
A 55-year-old woman presents with symptoms of “burning” eye discomfort, light sensitivity, and occasional pain that has been recalcitrant to aggressive lubrication and topical steroids. Visual acuity (VA) with spectacle correction is 20/20 in both eyes. There is mild MGD in both sets of eyelids, but the rest of the ocular and eyelid examination is within normal limits. Schirmer testing is 7 mm bilaterally.
DR. SHEN. I follow a variation of the DEWS II diagnosis and treatment algorithm. In my EHR, I have added a list of questions to ask about symptoms of nocturnal lagophthalmos (dry eye worse upon awakening in the morning) and to rule out other types of ocular surface disease (OSD; e.g., autoimmune diseases such as graft-vs.-host disease or rosacea, lagophthalmos, and recurrent or past severe eye infection) or mechanical reasons for poor blink function (e.g., Parkinson disease, cosmetic surgery, or use of Botox or fillers).
This patient has an Ocular Surface Disease Index (OSDI) score of 66, so I have a baseline to compare to future visits. Neither fluorescein nor lissamine green stain reveals any significant findings on the cornea or conjunctiva. With a thorough slit-lamp exam, I always evert the eyelids to look for other OSD culprits. This patient shows no staining, foreign bodies, concretions, scarring, or papillary or follicular conjunctivitis. To check IOP, I have the technician use an iCare tonometer to avoid instilling anesthetic that could affect the staining. Bilateral corneal sensation is confirmed by touching the cornea with a wisp of cotton. After I place topical anesthetic, the eye discomfort improves only 50% in both eyes.
With no ocular surface findings and severe burning pain symptoms, along with incomplete relief of pain with topical anesthetic, the DEWS II diagnostic algorithm indicates that the patient has either “symptoms without signs” or “neuropathic pain.” I follow the staged treatment steps outlined in DEWS II. (See below.)
The patient’s MGD is very common and is not a likely cause of her symptoms. Since it is easy, cheap, and low risk, I advise Step 1 treatment. I would also talk to the patient about neuropathic eye pain and how it differs from DED, reassuring her that, fortunately, I don’t see severe damage on the clinical exam. If there is access to laser in vivo confocal microscopy, corneal subepithelial nerve plexus imaging would likely show microneuromas, decreased nerve density, and increased tortuosity.1
I would also try DEWS II Step 2 therapy, low-dose topical steroids and punctal plugs, if the tear meniscus is low and there is no history of reflex tearing.
If plugs plus steroids did not help, I would then consider the following measures: autologous serum tears (AST) 20% every two hours for six months with low-dose steroids (I try to defer use of AST because of their out-of-pocket expense) and future placement of Prokera (biotissue); pain medications and referral to a pain specialist for the neuropathic eye pain; an FL41 filter (in case of light sensitivity) or moisture chambers; and lastly scleral lenses.
DR. MASSARO-GIORDANO. Neuropathic ocular pain has confounded ophthalmologists for many years. Many patients with neuropathic pain are dismissed by their doctors when, in fact, they are some of the most devastated patients and they need special care.
In addition to Dr. Shen’s list of medications to consider, I might add low-dose oral naltrexone 2 mg, an opioid antagonist.1 I would also note that, in my clinical experience, scleral lenses do not work as well if the pain has become centralized.
CASE 2: Exposure Keratopathy
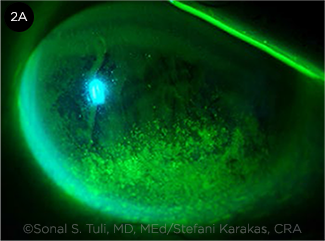 |
CASE 2. Diffuse inferior staining. Stefani Karakas, CRA, for Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa. Reprinted under a Creative Commons Attribution Noncommercial (CC BY-NC-ND 3.0) License.
|
An 89-year-old man with primary open-angle glaucoma presents with unilateral eye pain, redness, and fluctuating vision in the right eye. The other eye is not symptomatic. VA is 20/60 in the right eye and 20/25 in the left. He has had a trabeculectomy in the symptomatic eye. He uses a prostaglandin analogue and an alpha agonist in both eyes. Examination reveals mild generalized conjunctival injection, an elevated superior bleb without localized injection, and diffuse inferior corneal staining in the right eye. The elevated bleb causes incomplete eyelid closure. The left eye has scattered areas of punctate staining throughout the cornea. There is bilateral MGD. Schirmer testing with anesthesia is 12 mm in both eyes. TBUT is 6 seconds bilaterally.
DR. TULI. Glaucoma treatment and dry eyes have a well-established relationship, primarily because of toxicity from glaucoma drops. However, ocular surface changes caused by glaucoma surgery are now increasingly recognized. Both trabeculectomy and tube shunt surgery are associated with an elevated area near the limbus resulting from the filtering bleb or the patch graft over the tube. This elevation can lead to several problems with tear physiology. As with this patient, a high bleb can cause incomplete closure of the eyelid, resulting in exposure keratopathy.
Another reason for ocular surface instability is defective wiper action of the upper lid, leading to inadequate spread of the tear film, stagnation of tears under the bleb, and rapid TBUT (Fig. 2B). This dysfunction can also lead to the formation of dellen and filaments under the bleb.
Management of these patients is challenging. Bleb management early in the postoperative period is critical to minimize the risk of a very high and cystic bleb. In addition, patients with trabeculectomy blebs are advised against having procedures that may shorten the upper lid such as blepharoplasty and ptosis surgery.
Plugging the lacrimal canaliculi and having the patient use supplementary tears can increase the tear lake and mitigate the drying of the cornea in the area of exposure keratopathy. Patients are asked to use a lubricating ointment at night, when the exposure is particularly problematic. Moisture chamber glasses can help by improving the humidity of the air around the eye.
Filamentary keratitis is managed with debridement and hypertonic saline ointment at night or acetylcysteine eyedrops. However, patients with this condition often require surgical management to resolve the OSD. One strategy is to decrease the bleb height with compression sutures (Fig. 2C).
DR. MASSARO-GIORDANO. In my experience, switching patients to preservative-free single-use glaucoma drops can make a significant difference. Although these drops are more expensive, and often a prior authorization from the insurance company is required, I recommend making the extra effort.
I agree with telling patients to use lubricating ointment at night, though I prefer gels. If a patient’s eyes do not close, I recommend that they use disposable bubble eye bandages such as NitEye at bedtime.
Also, keep in mind that patients who need surgery might be candidates for microshunt glaucoma devices placed in the eye so there are no surface issues. Finally, be sure to treat the MGD to help the overall tear film.
CASE 3: Stain Without Pain
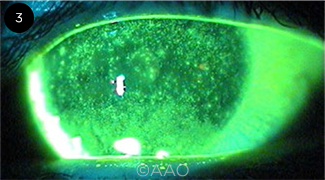 |
|
CASE 3. Diffuse corneal staining.
|
A 65-year-old man with facial rosacea and rhinophyma presents for routine examination, complaining of progressive reduced vision in both eyes over the last two years. He does not note any foreign body sensation. On exam, VA is 20/60 in both eyes. He has severe rosacea blepharitis with diffuse corneal staining bilaterally. TBUT is instantaneous in both eyes, and Schirmer testing is 8 mm bilaterally. He also has bilateral 2+ nuclear sclerotic cataracts and is considering cataract surgery.
DR. MASSARO-GIORDANO. Starting with the patient’s history, I ask whether he has a personal or family history of an autoimmune disorder. I ask about dry mouth, sleep apnea and CPAP use, and use of over-the-counter tears with preservatives, which can contribute to corneal staining. I also inquire about current medications, particularly hormonal therapy such as antiandrogens for prostate issues, as this can aggravate MGD.
I assess whether his reduced VA is due to surface disease and/or cataract by checking the refraction and seeing if it improves with correction. I look at the degree of rosacea and consider Demodex infestation, checking for debris on the lids and lashes, specifically waxy collarettes. I characterize the degree of telangiectasia and look at the expressible and nonexpressible meibomian secretions and whether the tears appear foamy.
Staining the conjunctiva and lid margins with lissamine green and looking for a widened Marx line are useful steps. I also carefully examine the tear height to rule out tear insufficiency dry eye (e.g., Sjögren syndrome). In addition, I would check corneal sensation; given the diffuse staining, one would expect some degree of symptoms.
Typically, I obtain LipiView images to look at incomplete blinks and thickness of the lipid layer as well as infrared images of the meibomian glands. I do MMP-9 testing as well, though I do not routinely get confocal images of the glands. However, I do obtain confocal images of the cornea if I suspect corneal nerve abnormality.
It’s critical to stabilize the tear film and treat inflammation before cataract surgery, even just to get accurate IOL calculations. My approach would start with aggressive lid hygiene. I instruct patients how to safely massage and clean their lids with premoistened pads. If I suspect Demodex, I recommend pads containing tea tree oil. If the patient has difficulty with this regimen, I may recommend LipiFlow and/or IPL treatment. I do recommend preservative-free artificial tears, but not necessarily lipid-containing tears, and a short course of antibiotic/steroid ointment for the eyes and lids. I consider azithromycin drops for the eyes and lids, brimonidine eyedrops (an off-label use currently being studied), and oral doxycycline or azithromycin. Adding a bedtime ointment or gel is helpful. If Demodex is severe, I add ivermectin cream or tablets.
I routinely discuss environmental triggers, such as heat, air vents, and fans, and I suggest use of goggles, humidifiers, etc. When outdoors, patients may benefit from wraparound sunglasses, with or without moisture chambers. Blinking exercises and appropriate tablet/computer use are also reviewed. If a patient asks about omega-3 fatty acids, I discuss the current evidence.2
It’s worth noting that rosacea is complicated, and its mechanisms are not clearly understood. There is some debate over whether more telangiectasia truly equals worse MGD, and my sense is that there is a correlation. A recent paper used lid injections of bevacizumab for MGD and saw good results with diminished vascularity.3
CASE 4: Post-LASIK Dry Eye
A healthy 38-year-old man who works as a financial analyst presents with fluctuating vision and foreign body sensation in both eyes. He underwent bilateral myopic LASIK six months ago. He is not using any drops. On exam, uncorrected VA is 20/25-1 in both eyes, improving to 20/20 with pinhole. LASIK flaps are unremarkable, but the corneas have moderate epithelial staining bilaterally. Schirmer testing is 6 mm, and TBUT is 8 seconds in both eyes. Corneal topography reveals a well-centered myopic LASIK, but the Placido rings are not crisp.
DR. SHEN. Based on the patient’s surgical history, I am most concerned about relatively neurotrophic post-LASIK dry eye. A refraction is performed to ensure he is not overminused or demonstrating presbyopia, but no glasses should be prescribed until his ocular surface is healed. I follow a variation of the DEWS II diagnosis and treatment algorithm and use a list of questions I keep in my EHR to rule out other OSD or mechanical reasons for poor blink function.
The patient’s OSDI score is 22. Lid eversion shows no staining, foreign bodies, concretions, scarring, or papillary or follicular conjunctivitis. Corneal sensation is markedly decreased in both eyes when tested before instillation of anesthetic. Topical anesthetic improves 100% of the mild foreign body sensation in both eyes.
LipiView I (Figs. 4A-4D) and Oculus Keratograph5 infrared meibography are performed. LipiView demonstrates a low lipid tear layer, worse in the right eye, and 100% incomplete blinks during the recording (partial blinks, PB). The Keratograph shows some shortening and atrophy of the meibomian glands as well as glands that are engorged with meibum. The patient and I discuss his incomplete blinks and the resulting relative lagophthalmos, compounded by long hours on the computer for his work, with incomplete blink leading to an unstable ocular surface.
Overall, I think the patient is neurotrophic with decreased corneal sensation from LASIK and has MGD compounded by incomplete blinks. It is hard to say why and when the incomplete blinking originated. It is seen commonly in current and previous contact lens wearers.
DEWS II Step 1 treatment for MGD is initiated. I find punctal plug occlusion helpful for incomplete blink and neurotrophic dry eye. The patient may also benefit from LipiFlow (Step 2). Since he is recently post-LASIK, I would recommend three-month dissolvable punctal plugs bilaterally in the lower lids, as his neurotrophic problems may improve with time. We would also discuss nighttime lubricating gel or ointment if symptoms are worse upon awakening.
DR. TULI. I completely agree with Dr. Shen’s diagnosis and management plan. The only difference in my approach is that I am a lot more aggressive with dry eye and MGD treatment, especially post-LASIK. I find that these patients have much more significant visual disturbances and are much more unhappy with their outcomes if they have dry eye. I would add higher-level MGD treatment with doxycycline and Restasis and also recommend longer-acting plugs. Since the patient is still symptomatic after six months, it suggests that his issues are likely to be long term. If the above treatments are not sufficient, I would also consider a short course of autologous serum tears or self-retaining amniotic membranes in these patients with a neurotrophic component to their dry eye.
Key Resources
For a comprehensive discussion of DED management, read the Academy’s Preferred Practice Pattern on Dry Eye Syndrome, updated in 2018, at aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp-2018. PDFs of the DEWS II publications and executive summaries that were published in The Ocular Surface in 2017 are available at the Tear Film and Ocular Surface Society website at www.tfosdewsreport.org.
|
CASE 5: Dry Eye Secondary to Sjögren Syndrome
A 54-year-old woman with a 20-year history of rheumatoid arthritis (RA) is referred by her rheumatologist for evaluation. She notes reduced vision and chronic discomfort bilaterally. She is currently using preserved artificial tears every hour without relief. She is on disease-modifying therapy for her RA, which is under good control systemically. On exam, VA is 20/40 in both eyes. There is virtually no tear lake. The cornea has diffuse staining with occasional filaments. Schirmer testing is 0 mm. The rest of the exam is unremarkable.
DR. ASBELL. This is classic dry eye disease with severe aqueous deficiency in the setting of a systemic immune-mediated disease, RA. Further examination would include documentation of the degree of OSD. For the cornea, this would entail vital dye testing with fluorescein using a Wratten #12 yellow filter handheld in front of the oculars as you observe the cornea with cobalt blue light to enhance visibility of the staining. For the conjunctiva, lissamine green would be used primarily to enable evaluation of response to treatment over time. The HD Analyzer can be helpful to distinguish between vision loss from OSD and cataract. The former is manifested by wave changes over time, between blinks, and the latter by a wave that is constant between blinks. It is likely that both surface disease and cataracts are contributing to reduced vision in this patient.
Although we have a pretty good idea why patients with Sjögren syndrome have dry eyes—inflammatory effects on the lacrimal gland—it is also good to look for MGD, which can contribute to surface changes and discomfort. It’s important to gently evert the lid to observe the puncta of the meibomian glands and, with slight pressure, look at the meibum: Are the glands obstructed? Is the meibum cloudy or pasty? If MGD is present, treat it in addition to treating the ocular surface directly. Check also for lid lag and exposure.
Unfortunately, with a Schirmer test of 0 mm, it is unlikely that stimulation will increase tears, so my efforts are mainly geared toward replacing the lubrication and keeping it on the eye. That said, I often do try immunomodulators, including topical cyclosporine or lifitegrast. Even these severe cases sometimes respond to low-dose topical steroid, such as nonpreserved dexamethasone 0.01% drops twice daily (compounded, off-label use). Unfortunately, systemic immunosuppression and/or modulation do not appear to be effective for ocular findings, likely because the lacrimal gland is already too severely damaged to respond.
For lubrication, only nonpreserved treatments are recommended. A thicker consistency, such as gels and ointments, may work better. Compounded autologous serum can be helpful, though strong evidence is sparse, and these drops can be costly and require careful attention to hygiene to avoid contamination. Punctal occlusion can be considered and may help keep the lubricants on the ocular surface for longer contact.
If filamentary keratitis is present, filament removal at the slit lamp can be a short-term fix and is especially helpful if lubrication is then maximized. Amniotic membrane with a bandage contact lens is sometimes useful for severe flare-ups, but it is not practical for this chronic condition. For both symptoms and vision, contact lenses may be needed. Occasionally, a soft bandage lens can help, but more typically scleral lenses, with their reservoir of fluid, are the only way to achieve clinical improvement.
DR. TULI. Sjögren syndrome patients can be very challenging, especially those who have an almost complete absence of aqueous tears. I explain to these patients that even preservative-free artificial tears should not be used every hour, as they can deplete the mucins that act as conditioners of the ocular surface to allow the tears to adhere to the eyes. In severe dry eye patients, secretagogue medications such as oral pilocarpine or cevimeline may be helpful—however, they stimulate salivary glands more than lacrimal glands and therefore work better for dry mouth. Finally, I stress the need to use topical cyclosporine chronically, even if patients do not perceive any benefit for their symptoms. The lacrimal gland damage that occurs in Sjögren syndrome is primarily due to lymphocytic infiltration, and the cyclosporine may mitigate further damage.
___________________________
1 Dieckmann G et al. Ophthalmology. 2017;124(11):S34-S47.
2 Dry Eye Assessment and Management Study Research Group. N Engl J Med. 2018;378:681-1690.
3 Jiang X et al. Drug Des Devel Ther. 2018;12:1269-1279.
DEWS II: Staged Management and Treatment Recommendations for Dry Eye Disease
Step 1
- Education regarding the condition, its management, treatment, and prognosis
- Modification of local environment
- Education regarding potential dietary modifications (including oral essential fatty acid supplementation)
- Identification and potential modification/elimination of offending systemic and topical medications
- Ocular lubricants of various types (if MGD is present, consider lipid-containing supplements)
- Lid hygiene and warm compresses of various types
Step 2
If above options are inadequate, consider:
- Nonpreserved ocular lubricants to minimize preservative-induced toxicity
- Tea tree oil treatment for Demodex (if present)
- Tear conservation
- Punctal occlusion
- Moisture chamber spectacles/goggles
- Overnight treatments (such as ointment or moisture chamber devices)
- In-office, physical heating and expression of the meibomian glands (including device-assisted therapies, such as LipiFlow)
- In-office intense pulsed light therapy for MGD
- Prescription drugs to manage DED1
- Topical antibiotic or antibiotic/steroid combination applied to the lid margins for anterior blepharitis (if present)
- Topical corticosteroid (limited duration)
- Topical secretagogues
- Topical nonglucocorticoid immunomodulatory drugs (such as cyclosporine)
- Topical LFA-1 antagonist drugs (such as lifitegrast)
- Oral macrolide or tetracycline antibiotics
Step 3
If above options are inadequate, consider:
- Oral secretagogues
- Autologous/allogeneic serum eyedrops
- Therapeutic contact lens options
- Soft bandage lenses
- Rigid scleral lenses
Step 4
If above options are inadequate, consider:
- Topical corticosteroid for longer duration
- Amniotic membrane grafts
- Surgical punctal occlusion
- Other surgical approaches (e.g., tarsorrhaphy, salivary gland transplantation)
IMPORTANT CAVEATS
- Potential variations within the disease spectrum are acknowledged to exist between patients, and the management options listed above are not intended to be exclusive. The severity and etiology of the DED state will dictate the range and number of management options selected from one or more steps.
- One or more options concurrently within each category can be considered within that step of the dry eye disease state. Options within a category are not ranked according to importance and may be equally valid.
- It should be noted that the evidence available to support the various management options differs and will inevitably be lower for newer management options. Thus, each treatment option should be considered in accordance with the level of evidence available at the time management is instigated.
___________________________
1 The use of prescription drugs needs to be considered in the context of the individual patient presentation, and the relative level of evidence supporting their use for that specific indication, as this group of agents differs widely in mechanism of action.
___________________________
SOURCE: Jones L et al. The Ocular Surface (2017):580e634.
|
Meet the Experts
Penny A. Asbell, MD, FACS Barret G. Haik Endowed Chair of Ophthalmology; director of the Hamilton Eye Institute; and professor of ophthalmology at University of Tennessee Health Science Center in Memphis. Disclosures: Allergan: C; Bausch + Lomb: C,S; Contact Lens Association of Ophthalmologists: C; MC2: C,S; Medscape: L; MioTech: C,S; NEI: S; Novartis: C,S; Oculus: L; Office of Dietary Supplements-NIH: S; Rtech: C,S; Santen: L; Scientia CME: L; Shire: C; Vindico: L.
Giacomina Massaro-Giordano, MD Codirector of the Penn Dry Eye & Ocular Surface Center; professor of clinical ophthalmology at the Scheie Eye Institute at the University of Pennsylvania in Philadelphia. Disclosures: Celularity: C; GlaxoSmithKline: C; PRN Physician Recommended Nutriceuticals: O.
Elisabeth M. Messmer, MD Professor of ophthalmology at the Ludwig-Maximilian University of Munich in Germany. Disclosures: Alcon: C,L; Allergan: C,L; Dompé: C,L; Santen: C,L; Shire C,L; Sun Pharmaceuticals: C; Thea: C,L; TRB-Chemedica: C,L; UrsaPharm: L; Visufarma: C,L.
Joanne F. Shen, MD Assistant professor of ophthalmology, chair of ophthalmology, and director of the Dry Eye Clinic at the Mayo Clinic in Arizona. Disclosures: None.
Sonal S. Tuli, MD, MEd Chair of ophthalmology at the University of Florida, Gainesville. Disclosures: None.
See the disclosure key at www.aao.org/eyenet/disclosures.