By Gabrielle Weiner, interviewing Anat Galor, MD, MSPH, Deborah S. Jacobs, MD, and Roy C. Levitt, MD
Download PDF
A patient walks into your office complaining of chronic dry eye symptoms but shows no staining or other signs of tear deficiency or hyperevaporation. Unsure of a diagnosis, you initiate treatment with topical therapies to optimize the ocular surface. The patient finds no relief. Now what?
Looking Beyond the Surface
Over the past decade, a new understanding has emerged: Some patients classified as having dry eye disease (DED) may not, in fact, have a problem with moisture on the ocular surface. Rather, they have a disruption of homeostasis in the nervous system.1
Process of sensitization. The nerve endings in the cornea become “sensitized” to normal environmental stimuli, perhaps from injury during refractive surgery or from a systemic disease. This sensitization resets the sensory thresholds too low, causing the biological alarm to trigger prematurely and produce symptoms of DED, despite a normal tear film. Inflammation from increased nerve activation further ramps up pain signaling.
Theoretically, plumping up the tear film should keep this dry eye alarm in check, reducing nociceptive pain—that is, nociceptor activation caused by stimuli at the ocular surface. But dysregulation of the ocular sensory apparatus, including central nervous system extensions, becomes entrenched over time, rendering surface treatments ineffective at managing the patient’s pain.2
At this point, the problem lies in the nervous system itself and is called neuropathic ocular pain. It also is referred to as corneal neuralgia or keratoneuralgia.3
 |
|
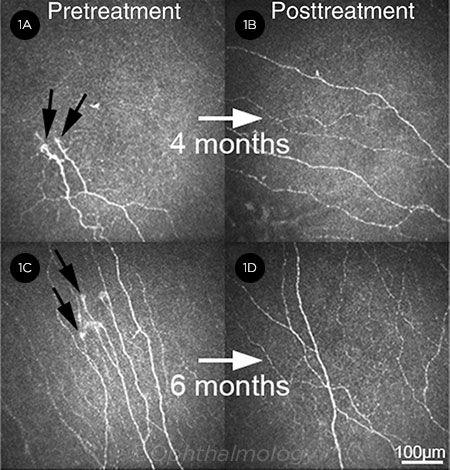
IMPROVEMENT. Before treatment (1A, 1C), microneuromas (black arrows), decreased nerve density, and increased tortuosity are evident in 2 patients. Following 4 and 6 months of treatment with autologous serum tears and low-dose anti-inflammatory therapy (1B, 1D), subbasal corneal nerve density is increased, and microneuromas are no longer present.
|
A Challenging Dx
“Pain without stain” is primarily a clinical diagnosis made for patients with corneal pain that has few-to-no clinical signs and is minimally, if at all, relieved by conventional treatments for DED.4
Signs and symptoms. Currently, there are no standard clinical criteria and no ocular sensory tests that are diagnostic. Anat Galor, MD, MSPH, at Bascom Palmer Eye Institute in Miami, looks for the specific symptom profile of burning and pain associated with wind and light. She also looks for signs that tend to be associated with neuropathic pain, such as persistent pain after anesthesia (topical proparacaine), which has emerged as a marker of “central sensitization” or centralized neuropathic pain.5
Roy C. Levitt, MD, a neuroanesthesiologist at the University of Miami Miller School of Medicine, added that patients with neuropathic pain tend to have more severe and chronic symptoms compared to patients with other subtypes of DED.
Comorbidities associated with neuropathic pain—such as anxiety, depression, fibromyalgia, and migraine—are also common in patients with chronic ocular pain.
Need for awareness. “In the absence of staining and responsiveness to treatments, busy doctors tend to say the eyes are fine and may dismiss complaints as psychosomatic or hysterical,” cautioned Deborah S. Jacobs, MD, at the Massachusetts Eye and Ear in Boston.
“Our practices are set up to deal with signs, not symptoms, and it is difficult to diagnose neuropathic pain because it is not concrete,” she acknowledged. That said, Dr. Jacobs pointed out that “you’re doing the patient a favor by spotting a potential nerve problem early—the earlier, the better, before the central pain pathways become sensitized.”
As the cornea is the most exposed mucosal tissue in the body and has the highest density of nociceptors of any tissue in the body, it is particularly vulnerable to dysregulation.3 “Our highest priority is educating ophthalmologists that dry eye can represent a ‘chronic overlapping pain condition’ that is best managed with a multidisciplinary approach,” Dr. Galor said.
Overarching Treatment Approach
Pain management is a field with no simple solutions, according to Dr. Jacobs. She recommends several macro-level strategies:6
Label the condition as a nerve problem. When communicating with patients about neuropathic pain, it is helpful to describe the condition as a nerve problem rather than an eye problem. “Patients typically appreciate the distinction,” said Dr. Jacobs. Reassure the patient that there is no blinding process occurring with the pain—that the nerves are sending false alarms.
Set realistic expectations. Both clinicians and patients need to recognize that there is no silver bullet to eliminate the pain. A combination of local, systemic, and psychological therapies provides the most benefit.
Schedule frequent visits. “Pain makes patients anxious; anxious patients are more susceptible to pain,” said Dr. Jacobs. Setting up office visits at 4-to 8-week intervals helps eliminate panicked visits and gives you a chance to assess and reassure the patient. You can increase the interval as the patient improves.
Collaborate with other clinicians. Dr. Levitt—who is not only a neuroanesthesiologist but also a pain physician and geneticist—emphasized the importance of collaborative care, especially when multimodal systemic approaches are needed. Depending on the patient’s comorbidities, for instance, you might work with pain specialists, psychiatrists, neurologists (especially when migraines are present), or oral surgeons (if temporomandibular joint disorder is present). Typically, these clinicians do not have experience assessing ocular endpoints, making ongoing ophthalmic care imperative.
Dr. Galor further emphasized, “Pain specialists have years of research behind them, and ocular pain with a neuropathic component resembles neuropathic pain elsewhere in the body. The key is not to reinvent the wheel! Let’s work with pain specialists and try what’s already available.”
Specific Treatment Options
The goal of treatment is to reduce pain signaling, not only to provide relief for the patient but also to prevent peripheral signaling from converting to centralized pain.4 Ophthalmic data for treating neuropathic pain are not strong, according to Dr. Levitt, so the following recommendations are based on available anecdotal, scientific, and preliminary clinical data in dry eye patients as well as evidence-based approaches from other neuropathic pain conditions. This is particularly true for systemic agents.
Local support and protection. The first step is to provide support and protection of the ocular surface and nerves. This may include lubricants (whichever one the patient likes best), punctal occlusion, goggles/glasses, bandage soft lenses, and the use of scleral lenses or PROSE (Prosthetic Replacement of the Ocular Surface Ecosystem).
Topical suppression of inflammation. A low-dose topical steroid can downregulate local inflammation. Other options include topical cyclosporine (Restasis), lifitegrast (Xiidra), and topical nonsteroidal anti-inflammatories (NSAIDs), although their benefit is uncertain.
Topical suppression of peripheral sensitization. Topical analgesics have not proven safe or effective for long-term treatment of ocular pain but may be helpful in the short term.
Other support of local nerve recovery. Autologous serum tears (Fig. 1) and amniotic membrane can be used to promote recovery of nerve structure and function.
Systemic suppression of central sensitization. To block the “learning” of pain, the following systemic agents can be used:
Gabapentin and pregabalin. Gabapentin is FDA-approved for postherpetic neuralgia (PHN) and epilepsy. Pregabalin is approved for PHN, fibro-myalgia, diabetic neuropathy, and certain seizure disorders.
Tricyclic antidepressants. Although tricyclic antidepressants are not labeled for neuropathic pain, a substantial body of literature supports their usefulness.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). SNRIs are a class of antidepressants known to produce analgesia, while serotonin reuptake inhibitors (SRIs) do not. Duloxetine, an SNRI, is approved for depression/anxiety, peripheral neuropathy, and fibromyalgia.
Other antiepileptics. These include carbamazepine, lamotrigine, and topiramate.
Opioids. The opioids tramadol and low-dose naltrexone are additional options.
Suppression of peripheral sensitization. Oral NSAIDs (e.g., diclofenac, ibuprofen, and naproxen); antiepileptic agents (e.g., gabapentin and pregabalin), and analgesic antidepressants (e.g., duloxetine and nortriptyline) are good choices, according to Dr. Levitt.
Bottom line on systemic drugs. Although some ophthalmologists are uncomfortable prescribing systemic drugs, these agents can be helpful when a patient has been suffering for many months (after this much time, the pain has likely centralized) or when a patient has history of a pain syndrome in other areas of the body (also suggestive of a central process). “Gabapentin, pregabalin, and antidepressants have excellent safety profiles. In some patients, systemic agents will transform their lives,” said Dr. Levitt.
Other Treatment Options
Omega-3 fatty acids. Dr. Jacobs is a proponent of omega-3 fatty acid supplementation to help reverse inflammation surrounding and sensitizing sensory nerves, despite the recent negative result from the DREAM study, which evaluated a much broader group of patients than only those with neuropathic “dry eye.”7 Often patients don’t take enough to see a benefit. Two to 3 grams per day are necessary, she said.
Stimulation treatments. Acupuncture, transcutaneous electrical nerve stimulation, transcranial magnetic stimulation, and transcranial direct current stimulation are used frequently in pain management. The rationale is that these treatments interrupt learned pain pathways, enhancing central “gating,” Dr. Galor said.
Psychological support. Data show that cognitive-behavioral therapy can be helpful for people with chronic pain syndromes.8 And other physicians, such as psychiatrists and internists, can play an integral role in pain management by prescribing the appropriate anxiolytic drugs and analgesic antidepressive drugs. The latter category does not include selective SRIs, such as fluoxetine and sertraline, which are not effective for neuropathic pain.
Nerve blocks. In severe cases, Drs. Galor and Levitt use nerve blocks to manage pain. They have 2 protocols: 1) botulinim toxin and 2) a combination of a long-acting anesthetic and steroids. Botulinim toxin A (BoNT-A) is already used to treat migraine. “When I talk to neuro-ophthalmologists, they talk about migraine pain and photophobia, and I talk about sensations of ocular dryness and photophobia with headaches. Turns out, we’re really talking about the same patients,” said Dr. Galor.
BoNT-A appears to have an effect on calcitonin gene-related peptide, which is part of the pathophysiology of migraine pain, so the team at University of Miami started following the exact same protocol used for migraine pain and had good results (see also “Botox Effective for Dry Eye and Photophobia,” News in Review, October).
When they do sensory blocks in patients who don’t suffer migraines, they use the anesthetic-steroid combination injections to block the periocular nerves (supraorbital, supratrochlear, infraorbital, infratrochlea). The effect of these blocks may last hours to months; thus, repeat injections are often needed.
___________________________
1 Belmonte C et al. Ocul Surf. 2017;15(3):404-437.
2 Galor A et al. Eye. 2015;29(3):301-312.
3 Goyal S, Hamrah P. Semin Ophthalmol. 2016;31(1-2):59-70.
4 Rosenthal P et al. Ocul Surf. 2009;7(1):28-40.
5 Crane AM et al. Br J Ophthalmol. 2017;101(9):1238-1243.
6 Jacobs DS. Curr Ophthalmol Rep. 2017;5(4):271-275.
7 Asbell PA et al., for the Dry Eye Assessment and Management Study Research Group. N Engl J Med. 2018;378(18):1681-1690.
8 Castelnuovo G et al. Front Psychol. 2016;7:115.
___________________________
Dr. Galor is staff physician at the Miami VA Medical Center and associate professor of ophthalmology at Bascom Palmer Eye Institute in Miami. Relevant financial disclosures: None.
Dr. Jacobs is associate professor of ophthalmology at Harvard Medical School. She is also faculty on the Cornea Service and director of the Ocular Surface Imaging Center at Massachusetts Eye and Ear in Boston. Relevant financial disclosures: None.
Dr. Levitt is professor and vice chair of translational research and academic affairs in the Department of Anesthesiology, Perioperative Medicine, and Pain Management at the University of Miami Miller School of Medicine. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Galor Allergan: C; Novaliq: C; Shire: C.
Dr. Jacobs BostonSight: E (through February 2018); Simple Contacts: C; TecLens: C.
Dr. Levitt Onspira Therapeutics: C,O.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|