Ophthalmology
Effects of Pupillary Dilation in PAC Suspects With Cataract
Brolucizumab Outcomes at 96 Weeks
OCT Retinal Thickness Map Detects HCQ Retinopathy
Ophthalmology Retina
Return to the OR After Vitrectomy for Vitreous Opacities
American Journal of Ophthalmology
Minimally Invasive Surgery for Intraconal Orbital Tumors
RVO Raises the Risk of Dementia
JAMA Ophthalmology
Sensory Impairment Is Linked to Perceived Discrimination
Visual Outcomes of Children With Optic Neuritis
Vitamin D3, Omega-3 Fatty Acids, and AMD Risk
Other Journals
Diagnosing Pediatric Orbital Cellulitis: CT or rMRI?
Thrombolysis for Central Retinal Artery Occlusion
IRIS Registry Snapshot: Switching Anti-VEGF Drugs in DME
Diabetic macular edema (DME) is a leading cause of vision loss in patients with diabetic retinopathy and can appear at any stage of the disease. Current treatment involves intravitreal injections of anti-VEGF agents; ophthalmologists typically start with one drug and switch to a different agent when the clinical response is suboptimal.
Verana Health assessed switching patterns by ana-lyzing statistically de-identified electronic health record data from the Academy’s IRIS (Intelligent Research in Sight) Registry. A switcher was defined as an eye that had at least three injections of one agent and then had a different agent at a subsequent visit. Eyes without specified laterality were excluded.
Of the 202,532 treated eyes with DME, 19,083 (94.2%) were switched to another anti-VEGF drug between Jan. 1, 2016, and Dec. 31, 2019. Of these, 65.7% were switched to aflibercept.
Aflibercept and ranibizumab net gained 9,325 and 1,613 eyes, respectively, whereas bevacizumab lost 10,941 eyes to switching.
Aflibercept and ranibizumab gained most of their switches from bevacizumab (83.1% and 63.7%, respectively).
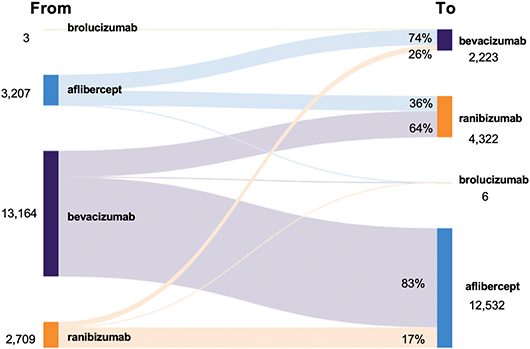 |
SANKEY DIAGRAM. Switching patterns of anti-VEGF agents in DME.
|
___________________________
Note: The Academy has partnered with Verana Health to curate and analyze IRIS Registry data.