By Lori Baker-Schena, MBA, EdD, interviewing Penny A. Asbell, MD, MBA, Edward J. Holland, MD, and Bennie H. Jeng, MD, MS
Download PDF
With the approval of lifitegrast (Xiidra), clinicians now have another prescription option in addition to cyclosporine (Restasis) for treating dry eye disease (DED).
Lifitegrast’s introduction “is noteworthy for 2 key reasons,” said Bennie H. Jeng, MD, MS, at the University of Maryland School of Medicine in Baltimore. To begin with, he said, the drug is “the first new medication in 13 years to be approved for patients with dry eye disease.” Secondly, it is the only prescription eyedrop that has an approved indication to treat both the signs and symptoms of DED.
Moreover, said Penny A. Asbell, MD, MBA, at Icahn School of Medicine at Mount Sinai in New York, lifitegrast’s mechanism of action confirms “the role of inflammation as a core part of the pathogenesis” of DED.
The Question of Inflammation
“Dry eye disease is complex because it varies in clinical presentation and can be caused by a variety of factors,” said Edward J. Holland, MD, at the University of Cincinnati.
Although the underlying cause of DED is not fully understood, researchers have found that inflammation of the ocular surface and lacrimal gland plays a key role in pathogenesis.1 In a widely cited 2004 study, Stephen C. Pflugfelder, MD, presented evidence linking inflammation and dry eye.2 In 2014, Dr. Asbell and her colleague Yi Wei, PhD, conducted an extensive literature review that “clearly demonstrated inflammation as a core mechanism through basic, translational, and clinical research.”1
Different pathways. Although both cyclosporine and lifitegrast target the inflammation involved in DED, they act on different inflammatory pathways.
In his research, Dr. Plugfelder observed “an increase in cytokines and proteases in the tear fluid, adhesion molecule expression by the conjunctival epithelium, and T-cell infiltration of the conjunctiva” in dry eye patients.2 Cyclosporine reduces inflammation by acting as a “potent inhibitor of T-cell proliferation and thereby inhibiting T-cell–mediated immune responses” responsible for causing dry eye.3
In contrast, lifitegrast is a small-molecule integrin antagonist that “inhibits T-cell inflammation by blocking the binding of 2 important cell surface proteins (lymphocyte function–associated antigen 1 [LFA-1] and intercellular adhesion molecule 1 [ICAM-1]), thus lessening overall inflammatory response,” the authors of a 2016 study reported.4 In essence, lifitegrast inhibits the LFA-1/ICAM-1 interaction, which in turn is thought to block the subsequent cycle of T-cell–mediated inflammation.4
Furthermore, research on lifitegrast as an ophthalmic solution to treat DED demonstrated that the drug is able to strongly inhibit T-cell adhesion to ICAM-1–expressing surfaces. It also has “high solubility in aqueous media; rapid absorption into ocular tissues; rapid clearance from systemic circulation; and a good in vitro and in vivo safety profile,” the 2016 study authors wrote.4
 |
|
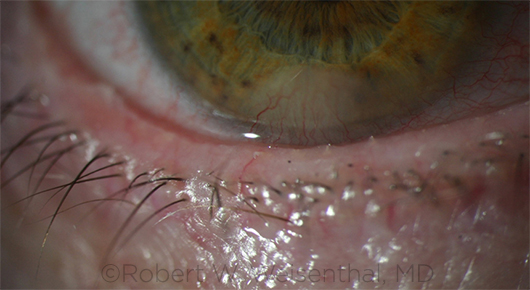
RESEARCH TARGET. Studies continue to evaluate the role of meibomian gland dysfunction (shown here), as it can cause or exacerbate dry eye symptoms.
|
The Road to Approval
“After Restasis gained approval in 2003 by showing improved Schirmer test results, the FDA mandated that any candidate dry eye drug had to demonstrate statistical significance in the reduction of both a sign and a symptom of dry eye and then reproduce the results in another study,” said Dr. Holland.
“Achieving significance in a sign and symptom in the same trial is not an easy undertaking, which may be why 20 new drug applications failed to obtain FDA approval after Restasis came on the market,” Dr. Holland added.
Shire (the maker of lifitegrast) took a different approach, Dr. Holland said. Instead of trying to demonstrate clinical efficacy in both signs and symptoms of DED in the same study, the company conducted 4 randomized double-masked placebo-controlled trials, each 12 weeks long, that involved more than 2,100 patients. Two of the trials—a phase 2 study and the phase 3 study OPUS-1—evaluated signs of DED, while the phase 3 studies OPUS-2 and OPUS-3 targeted DED symptoms.
In the phase 2 study and OPUS-1, the symptoms of patients who received the drug improved in 4 to 6 weeks, Dr. Holland said. And “in the OPUS-2 and OPUS-3 trials, we observed that lifitegrast significantly improved symptoms as early as day 14,”5 he added. “This is much quicker than relief from cyclosporine and could represent a key benefit in treating patients with DED.”
Dr. Holland explained that while the OPUS-3 trial was designed to evaluate symptom effects in patients with moderate to severe DED, the signs were included as part of the safety assessment. “Positive trends” were observed for conjunctival redness score, corneal fluorescein staining score, conjunctival lissamine green staining, and Schirmer tear test with lifitegrast versus the placebo group.5 In addition, lifitegrast was well tolerated, and no serious ocular adverse events were noted.
In the Office
While Drs. Asbell and Jeng expressed enthusiasm about the introduction of a new alternative for their patients with DED, they are both approaching lifitegrast with caution as they carefully consider their patient populations.
“Restasis is low-dose cyclosporine and works for a large majority of patients who have mild to moderate dry eye,” said Dr. Jeng. “However, working in a tertiary care facility, I see patients who present with very severe dry eye problems, and these individuals may benefit from lifitegrast. Patient selection is crucial, and we must proceed cautiously.”
Dr. Jeng added, “We need to look closely at the inclusion criteria and be careful how we extrapolate the data. For example, at this point I am not sure if my graft-vs.-host disease [GVHD] patients with dry eyes are candidates for lifitegrast. I need more information.”
Dr. Asbell, who is also exercising caution, noted that research findings do not always mimic clinical practice. However, she is encouraged by the study data supporting the anti-inflammatory properties of lifitegrast, specifically those data indicating that the drug seems to prevent the lymphocytes from reaching the ocular surface, thus decreasing the overall inflammatory response. This may benefit patients with Sjögren syndrome, a systemic chronic inflammatory disorder (see “Guidelines for Sjögren Syndrome,” below).
Although no head-to-head studies have been conducted of cyclosporine and lifitegrast, patients who experience burning with cyclosporine may be good candidates for lifitegrast. The data suggesting that lifitegrast may have a more rapid effect may also make it a preferable choice for some patients, said Dr. Asbell.
“Patients with dry eyes represent a diverse group, so not all patients will react to lifitegrast the same way,” Dr. Asbell said. “Some [ophthalmologists] have suggested conducting studies of lifitegrast with a better-defined group—for example, patients with GVHD. There is no doubt that the availability of lifitegrast is exciting; now we have to see how effective it will be in everyday care. Careful patient selection is key.”
Guidelines for Sjögren Syndrome
Managing dry eye disease is complex, especially in patients with Sjögren syndrome. In 2015, Dr. Asbell coauthored a comprehensive paper providing clinical guidelines based on published treatments.1
Using this information, the Sjögren Syndrome Foundation (SSF) developed its own clinical practice guidelines for patients with the disease.2
The suggested treatments are wide ranging and start with tear replacement with artificial tears or lubricant solutions for early DED, followed by dietary supplements (omega-3 essential fatty acids), anti-inflammatory measures, and oral secretagogues (such as cevimeline and pilocarpine). Other treatment options include punctal plugs and topical antibiotics or systemic doxycycline therapy to control meibomian gland disease.
Lifitegrast’s role in treating these patients has yet to be determined, but the drug holds promise given its anti-inflammatory properties, Dr. Asbell said.
While the foundation’s guidelines are an excellent resource for ophthalmologists who treat Sjögren patients, the use of a blood test to identify early markers of Sjögren is also worth considering, Dr. Asbell said. “Sjögren is historically difficult to diagnose, and [the diagnosis] often takes years. With the development of this blood test, we have the opportunity to make the diagnosis earlier, which can lead to earlier intervention.”
___________________________
1 Foulks GN et al. Ocul Surf. 2015;13(2):118-132.
2 Sjögren Syndrome Foundation. Ocular Management in Sjögren’s Patients, May 12, 2016. Accessed Dec. 1, 2016.
|
Future Research
One of the challenges for researchers looking to bring new dry eye treatment to market is the lack of minimally invasive objective metrics to quantify the condition. “One of the reasons we have so many cholesterol medications is because we have concrete metrics to study their effect,” Dr. Asbell said. “Not so in dry eye diseases. Instead, we ask patients how they feel. And even with corneal fluorescein staining, it really is up to the observer in terms of rating the test.”
Dr. Asbell is the study chair of the Dry Eye Evaluation and Management (DREAM) clinical trial. The NIH-funded trial has 2 purposes: studying the effectiveness of omega-3 fatty acid supplements in relieving dry eye symptoms and identifying inflammatory biomarkers to shed new light on the mechanisms of action that lead to DED.
“We anticipate gathering a large amount of data, which we hope will allow us to discover a minimally invasive method of measuring the severity of dry eye and how the condition responds to treatment,” she said.
Other DED researchers are focusing their attention on imaging the ocular surface and taking a closer look at the role of meibomian gland dysfunction.
While Dr. Asbell predicts that lifitegrast is just the beginning of a growing number of treatment options for DED, the condition will continue to present multiple challenges in the meantime, she said. As an example, she cited patients who opt for premium intraocular lenses following cataract surgery. “You can perform a perfect operation with excellent results, but if the patient subsequently complains about his dry eyes, and he is upset that he paid for a premium lens and is now unhappy, dry eye becomes a quality-of-life issue for that patient—and his surgeon. This is why it is imperative to continue to identify novel ways to measure and treat DED.”
___________________________
1 Wei Y, Asbell PA. Eye Contact Lens. 2014;40(4):248-256.
2 Pflugfelder SC. Am J Ophthalmol. 2004;137(2):337-342.
3 Schultz C. Ophthalmol Eye Dis. 2014;6:37-42.
4 Perez VL et al. Ocul Surf. 2016;14(2):207-215.
5 Holland EJ et al. Ophthalmology. 2017;124(1):53-60.
___________________________
Dr. Asbell is professor of ophthalmology and director of the cornea service and the Refractive Surgery Center at Icahn School of Medicine at Mount Sinai in New York, N.Y. Relevant financial disclosures: Shire: C.
Dr. Holland is director of cornea services at the Cincinnati Eye Institute and professor of ophthalmology at the University of Cincinnati. Relevant financial disclosures: Shire: C, L.
Dr. Jeng is professor and chair of ophthalmology and visual sciences at the University of Maryland School of Medicine in Baltimore. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.