Download PDF
In a small randomized controlled trial, production of aqueous tears and secretion of goblet cells increased following application of the Oculeve Intranasal Lacrimal Neurostimulator (Allergan).1 This handheld device works by delivering electrical stimulation to the anterior ethmoidal branch of the trigeminal nerve in the nasal cavities. It potentially offers a new mechanism for treating dry eye disease—now largely managed with artificial tears and anti-inflammatory agents.
Methods and results. In the study, 10 patients with dry eye and 5 without underwent a screening visit and 2 treatment visits, randomized to either sham extranasal treatment or active intranasal treatment with the device. The researchers measured tear meniscus height using anterior segment optical coherence tomography. In addition, they applied an impression membrane to the surface of the conjunctiva to measure the ratio of degranulated to nondegranulated goblet cells as a marker of secretion. Measurements were taken before and after applications.
In patients with and without dry eye, treatment with the ILN significantly increased tear volume and effectively stimulated goblet cells, which produce stabilizing mucus in tears and are pivotal for maintaining tears’ health and stability, said Stephen C. Pflugfelder, MD, from the Baylor College of Medicine in Houston. Results were better when compared both with baseline measurements and with the results of the sham extranasal treatment.
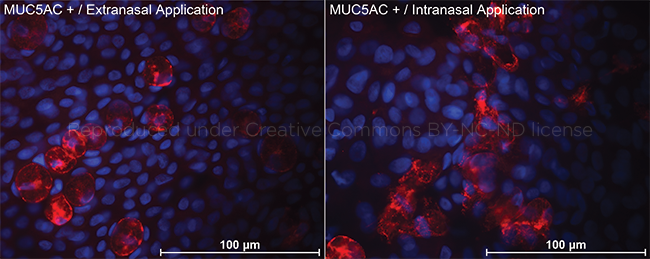 |
SHAM OR ACTIVE TREATMENT. Mucin 5AC–stained impression cytology (×340). (Left) After sham application, all goblet cells appear to be in a nondegranulated form, with intact boundaries and mucous granules remaining in the cells. (Right) After active treatment, most of the goblet cells showed a degranulation pattern with disruption of cell morphology and release of mucous granules and mucin outside the cells.
|
Impact for patients. “These are encouraging results,” said Dr. Pflugfelder, “especially because this may make it possible to use patients’ own neural pathways to stimulate tears without relying on a pharmacologic approach.” However, he added, there might be a nice synergy in using it with cyclosporine (Restasis) to increase the density of goblet cells, followed by Oculeve to stimulate their release.
Although this study did not evaluate symptom relief, a pilot study of 40 patients using the device 4 times daily reported a significant reduction in dry eye symptoms at each follow-up visit.2
Allergan has applied for FDA approval for the device, said Dr. Pflugfelder, and expects approval as soon as the third quarter of this year.
—Annie Stuart
___________________________
1 Gumus K et al. Am J Ophthalmol. 2017;177:159-168.
2 Friedman NJ et al. Clin Ophthalmol. 2016;10:795-804.
___________________________
Relevant financial disclosures—Dr. Pflugfelder: Allergan: C.
For full disclosures and disclosure key, see below.

More from this month’s News in Review