Download PDF
In a pair of studies, a team of Chinese researchers has reported on long-term outcomes of gene therapy for Leber hereditary optic neuropathy (LHON)1 and elucidated some factors that predispose patients to better visual outcomes following treatment.2
Study No. 1: Seven years of follow-up. In a small study of nine patients who underwent gene therapy for LHON, two-thirds continue to have persistent, significant gains in their best-corrected visual acuity (BCVA) after seven years (range, 75-90 months).1
The scientists found that the improvement in BCVA in these patients was ≥0.3 logMAR, equivalent to 15 letters on the EDTRS chart, said principal investigator Bin Li, MD, PhD, at the Huazhong University of Science and Technology and Tongji Hospital, both in Wuhan, China. In addition, no adverse outcomes were noted. “This demonstrates the long-term safety and efficacy of gene therapy,” Dr. Li said.
Protocol and results. All patients received a single unilateral intravitreal injection of a recombinant adeno-associated virus serotype 2 (AAV2) carrying the ND4 gene, which is mutated in most LHON cases.
Of the six patients who maintained clinically significant improvement in BCVA, the vision of two improved enough that one was admitted to a high school and the other, who gained 8 lines of BCVA, was able to travel to Beijing to obtain a job one year after treatment, Dr. Li said.
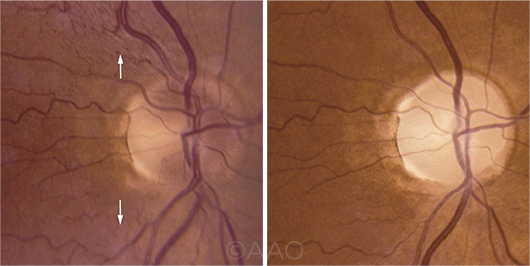 |
LHON. Genetic tests confirmed a point mutation in this patient with LHON. (Left) Arrows indicate peripapillary telangiectasias observed in the acute phase of vision loss. (Right) Several months later, the optic disc is diffusely pale.
|
Study No. 2: A look at treatment response. In the second study,2 the researchers reported on early treatment responses from a large prospective trial (149 subjects, including seven from Argentina). “On average, responders convert from legally blind to low vision, with a good percentage of patients reaching normal vision,” Dr. Li said. “We will publish the full results soon, and we are actively investigating why some patients responded significantly better than the others.”
The initial results in this study showed that about a third of the patients (28.9%) had significantly improved vision within three days of treatment, including significant improvement in acuity in some (18.8%) uninjected fellow eyes. These results confirm the promising findings from the smaller study, Dr. Li said.
The subjects who experienced such rapid acuity gains were the youngest patients and those who had the best vision before treatment, Dr. Li said. In addition, female participants had better responses to treatment than did their male counterparts, he said.
Need for additional investigation. Might patients benefit even more from a second injection? Dr. Li said his team is moving cautiously on this, because the first patient they treated received two injections and the visual outcome was poor.
“Years of research has made us realize that gene therapy is very different from small molecule and antibody drug treatment,” Dr. Li said. “The specific mechanism of action may extend beyond the simple gene replacement concept, and much more needs to be explored in order to fully unveil and leverage this new modality of therapy.”
An overview of the field. The Chinese group’s studies, which began with preclinical work in 2008, are the longest-running trials so far to report on using a recombinant AAV vector to deliver a normal ND4 mitochondrial gene to patients who have a mutant form of the gene. This mutation, G11778A, accounts for most cases of LHON and the severest disease.3
Another group, based at the University of Miami, reported in 2017 on using their own AAV vector containing the ND4 gene on 14 LHON patients, nine of whom had been followed for at least 12 months.4
A third AAV vector for delivering the ND4 gene is being investigated by European researchers on behalf of GenSight Biologics, which is based in Paris. The group has completed phase 1/2 clinical trials,3 and a phase 3 trial is ongoing.
—Linda Roach
___________________________
1 Yuan J et al. Ophthalmolology. Published online Feb. 25, 2020.
2 Liu HL et al. Acta Ophthalmol. Published online Feb. 24, 2020.
3 Bouquet C et al. JAMA Ophthalmol. 2019;137(4):399-406.
4 Guy J et al. Ophthalmolology. 2017;124(11):1621-1634.
___________________________
Relevant financial disclosures—Dr. Li: Wuhan Neurophth Biotechnology: S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Busch None.
Dr. Li Wuhan Neurophth Biotechnology: S.
Dr. Mansouri Alcon: L, Allergan: C,L; Bayer: S; Implandata: C; Glaukos: S; Novartis: L; New World Medical: C,L; Optovue: L,S; Santen: L,S; Sensimed: C; Thea: L; Topcon: L,S.
Dr. Sim Allergan: C; Bayer: C; Big Picture Eye Health: C; Haag Streit: C; Novartis: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review