By Xian Hui Lim, MBBS, Daniel S.W. Ting, MD, PhD, and Adrian Koh, MBBS, FRCS, MMED, FRCOphth, FAMS
Edited By: Ingrid U. Scott, MD, MPH, and Bennie Jeng, MD
Download PDF
Although there has been promising research into pharmacologic and other approaches that could slow the progress of retinitis pigmentosa (RP), no definitive treatment currently exists. Currently, the management of RP should involve a multidisciplinary approach, which may include pharmacologic therapy in some cases, as well as monitoring and treatment of associated complications and participation in occupational therapy and support groups. Genetic counseling and testing also play an important role; see the discussion of genetics and RP in last month’s Ophthalmic Pearls.
Pharmacologic Therapy
Vitamin A supplements. There is conflicting evidence on the use of vitamin A. Chatzinoff et al. found that vitamin A supplementation over three years did not improve visual acuity (VA), Goldmann visual fields, or dark adaptation threshold in patients with RP.1
In contrast, Berson et al. reported that patients receiving high-dose vitamin A supplementation (15,000 IU/day) had a slower reduction in electroretinogram (ERG) amplitudes of cone photoreceptors per year, but there was no significant difference in the decline of visual fields and VA compared with patients on trace (75 IU) amounts of vitamin A.2 However, the study design might have been limited in its sensitivity to detect subtle visual field deterioration, and the study duration (mean follow-up, 5.2 years) might have been too short to detect a correlating change in VA.3 A subgroup analysis of patients who had reliable visual field results, however, demonstrated a reduced rate of visual field among those patients on vitamin A supplementation.4
Risks of high-dose vitamin A supplementation include teratogenicity and a slightly higher risk of osteoporotic hip fractures.3 Annual monitoring of fasting serum vitamin A levels and liver function test studies are recommended.3
Vitamin A supplementation should be avoided in patients with autosomal recessive RP secondary to ABCA4 gene mutations. Apart from accounting for 3% of autosomal recessive RP, ABCA4 gene mutations also cause cone dystrophies, cone-rod dystrophies, and Stargardt disease.5 A study by Radu et al. observed that high-dose vitamin A supplementation resulted in more lipofuscin pigment accumulation in mice with knockout ABCA4 gene mutations than in wild-type mice. This accumulation leads to photoreceptor degeneration.5 It is plausible that a similar outcome could apply to humans with ABCA4 gene mutations.
Docosahexaenoic acid (DHA) supplements. Two randomized controlled trials by Hoffman et al. conducted over four years among patients with X-linked RP showed that, while safe, DHA supplements did not improve VA, ERG, or dark adaptation threshold results compared with placebo.6,7 However, there has been some indication of an inverse relationship between DHA concentration in red blood cells and retinal degeneration, as well as slower rates of visual field loss with higher dietary consumption of omega-3 fatty acids.3
Berson et al. looked at the combination of vitamin A and DHA supplementation and found no difference in the deterioration of VA, visual field, or ERG responses.8 A subgroup analysis compared patients taking vitamin A and placebo with those taking vitamin A and DHA. Findings showed that patients who had not been taking vitamin A supplements prior to the study had a statistically significant reduced mean annual rate of decline in visual field in the vitamin A and DHA group compared with the vitamin A and placebo group.9 Comparison of annual 30-Hz ERG amplitude decline revealed similar results: Patients not taking vitamin A before the study demonstrated significantly less ERG amplitude decline in the vitamin A and DHA group compared with the vitamin A and placebo group.9
Lutein supplementation. Lutein, a type of carotenoid obtained from dietary sources, contributes to the yellow pigmentation of the macula. In addition, it has antioxidant properties that protect the retina from reactive oxygen species, and it attenuates the damaging effects of lipofuscin pigments.10,11
Macular pigment optical density correlates linearly with the concentration of macular pigments such as lutein and zeaxanthin and has been found to be lower in eyes with retinal diseases such as age-related macular degeneration and Stargardt disease but not in those with RP.11 Nevertheless, given the protective role that lutein plays in the retina, supplementation has been studied as a form of treatment in RP.
In a randomized controlled trial conducted by Bahrami et al. over six months in 34 patients with RP, lutein supplements helped slow central visual field loss (assessed by static perimetry) compared with placebo.12 Berson et al. conducted another randomized controlled trial over four years in 225 patients with RP and found that lutein supplementation combined with vitamin A helped slow the average rate of decline of retinal sensitivity on Humphrey Field Analysis 60-4 testing, but the combination did not have any effect on VA, full-field cone ERG amplitude, or visual field on Humphrey Field Analysis 30-2 testing.13
CNTF intraocular implants. Ciliary neurotrophic factor (CNTF) has been shown in animal models and phase 1 studies to have a protective effect on retinal cells in the setting of photoreceptor degeneration.14 Two clinical trials conducted by Birch et al. examined the effects of different doses of encapsulated CNTF intraocular implants in patients with early and late RP.14 There was no significant difference in the best-corrected VA of patients in the high-dose versus sham and low-dose versus sham groups in either study.14 Both studies also found that patients with high-dose implants had decreased visual field sensitivity compared to those with sham implants at 12 months, although this difference became statistically insignificant six months after removal of the implant.14
Other agents. Other, smaller studies with promising findings investigated the use of beta-carotene acid derived from Dunaliella bardawil algae, oral valproic acid, and oral nilvadipine treatment in patients with RP. However, controlled studies with larger sample sizes are needed to corroborate these results.15
 |
|
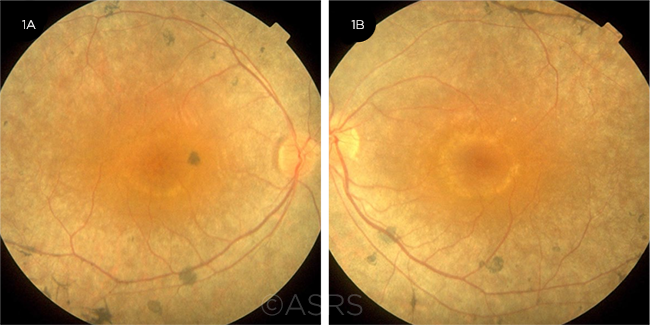
RP AND CME. Fundus photos of the right (1A) and left (1B) eyes of a 30-year-old woman with CME secondary to RP. This image was originally published in the ASRS Retina Image Bank. Hamid Ahmadieh, MD. Cystoid Macular Edema due to Retinitis Pigmentosa. Retina Image Bank. 2017; Image Numbers 7680 (left eye) and 7681 (right eye). © The American Society of Retina Specialists.
|
Nonpharmacologic Approaches
Light protection. Retinal degeneration is partly light dependent in some genetic types of RP, so strategies of light protection are hypothesized to help in RP.15 Two animal studies found that constant darkness decreased the rate of photoreceptor degeneration, but case studies of two patients with RP who occluded one eye or pupil for prolonged periods found similar severities of RP in both occluded and uncovered eyes.3
Hyperbaric oxygen delivery. Vingolo et al. conducted two studies on hyperbaric oxygen therapy in RP. In the first of these, the researchers observed that 11% of patients who underwent hyperbaric oxygen therapy experienced an improvement in low-noise ERG, with no worsening observed in any patients. In the control group, none of the subjects showed improvement in ERG results, while 62% experienced worsening.16
The second study compared hyperbaric oxygen therapy with vitamin A supplementation and demonstrated better ERG b-wave amplitudes and greater preservation of VA and visual field in the group receiving hyperbaric oxygen delivery.17 However, these positive results should be considered within the limits of some undisclosed data and change of equipment during the study.15
Retinal prostheses. The Argus II Retinal Prosthesis System (Second Sight Medical Products) involves a retinal implant approved for use in the United States and Europe. It can provide a basic form of navigational vision in patients with very advanced RP.18 Several other retinal prostheses are in development, as is an implanted cortical stimulation device.
Refraction, occupational therapy, and low vision support. Any refractive error present should be corrected. Other measures that patients with RP may find helpful in coping with their vision loss include participation in vision rehabilitation clinics and the use of visual aids such as magnifiers and night vision devices.3
In patients with advanced RP, it is important to ensure that appropriate referrals are made to occupational therapists and low vision clinics. Home modification and education on low vision aids help patients maximize their remaining functional vision. Support groups may also be beneficial to patients in managing psychosocial difficulties.
Treatment for RP Complications
RP can be associated with some complications that can be treated to help improve the patient’s visual potential.
Cataracts. Posterior subcapsular cataracts are seen in approximately half of patients with RP and may be surgically removed when significant enough to hinder vision.3
CME. Cystoid macular edema (CME) tends to be chronic in patients with RP (Fig. 1). Carbonic anhydrase inhibitors such as acetazolamide have been used at a daily dosage of 500 mg or less.19 Close monitoring is needed, as there is a risk of rebound intraretinal fluid accumulation with continued use.20 Similarly, topical dorzolamide has been successful in treating CME, although rebound effects have also been observed in some cases.19,21 Intravitreal or sub-Tenon injections of triamcinolone acetonide have been tried, but the effects have generally not been sustained.19
AIR. Autoimmune retinopathy (AIR) is a rare group of inflammatory conditions associated with the presence of antiretinal antibodies.22,23 It has been suggested that some cases of one of these conditions—nonparaneoplastic AIR (npAIR)—may occur secondary to retinal diseases such as RP with CME.23
Although the pathophysiology of npAIR remains undetermined, an expert consensus panel agreed that local or systemic steroid therapy and immunosuppression with antimetabolites or T-cell inhibitors should be used first for treatment.22 (For further information on AIR and npAIR, see Part 1 of this series in last month’s Ophthalmic Pearls.)
Future Directions
With the advent of genetic studies, many different treatment methods for RP are currently being explored. Therapies targeting the replacement or silencing of specific genetic mutations in RP are being studied. However, numerous genes and mutations are involved in RP; thus, other investigational modalities aim to deliver nutritional or neuroprotective factors to biochemical pathways. This could be potentially useful for a wider range of genetically diverse RP patients.3
Trials involving the transplantation of proteins, retinal pigment epithelium, photoreceptors, and stem cells are also under way.3,18 Another area of current research is the use of electrical devices to stimulate the retina, optic nerve, and visual cortex.3
___________________________
1 Chatzinoff A et al. Arch Ophthalmol. 1968;80(4):417-419.
2 Berson EL et al. Arch Ophthalmol. 1993;111(6):761-772.
3 Hartong DT et al. Lancet. 2006;368(9549):1795-1809.
4 Berson EL. Digit J Ophthalmol. 1998;4(7). www.djo.harvard.edu/site.php?url=/physicians/oa/377. Accessed March 10, 2020.
5 Radu RA et al. Invest Ophthalmol Vis Sci. 2008;49(9):3821-3829.
6 Hoffman DR et al. Am J Ophthalmol. 2004;137(4):704-718.
7 Hoffman DR et al. JAMA Ophthalmol. 2014;132(7):866-873.
8 Berson EL et al. Arch Ophthalmol. 2004;122(9):1297-1305.
9 Berson EL et al. Arch Ophthalmol. 2004;122(9):1306-1314.
10 Bernstein PS et al. Prog Retin Eye Res. 2016;50:34-66.
11 Bernstein PS et al. Vision Res. 2010;50(7):716-728.
12 Bahrami H et al. BMC Ophthalmol. 2006;6:23.
13 Berson EL et al. Arch Ophthalmol. 2010;128(4):403-411.
14 Birch DG et al; Ciliary Neurotrophic Factor Retinitis Pigmentosa Study Groups. Am J Ophthalmol. 2013;156(2):283-292.e1.
15 Sacchetti M et al. J Ophthalmol. 2015;2015:737053. doi: 10.1155/2015/737053.
16 Vingolo EM et al. Doc Ophthalmol. 1998-1999;97(1):33-39.
17 Vingolo EM et al. Graefes Arch Clin Exp Opthalmol. 2008;246(1):93-98.
18 Zhang Q. Asia Pac J Ophthalmol (Phila). 2016;5(4):265-271.
19 Parmeggiani F et al. Curr Genomics. 2011;12(4):250-259.
20 Apushkin MA et al. Retina. 2007;27(8):1112-1118.
21 Grover S et al. Am J Ophthalmol. 2006;141(5):850-858.
22 Fox AR et al. Am J Ophthalmol. 2016;168:183-190.
23 Braithwaite T et al. Ophthalmologica. 2012;228(3):131-142.
___________________________
Dr. Lim is an ophthalmology resident at the Singapore National Eye Centre. Dr. Ting is associate consultant at Singapore National Eye Centre and assistant professor at Duke-National University Singapore. Dr. Koh is the founding partner and senior consultant at the Eye & Retina Surgeons, Camden Medical Centre, associate professor at National University Singapore, and a visiting consultant to the vitreoretinal service at the Singapore National Eye Centre. Financial disclosures: None.
___________________________
LAST MONTH. See the June Ophthalmic Pearls for Part 1 of Retinitis Pigmentosa, covering the basics of genetics and natural history, as well as signs and symptoms, testing, and imaging.