By Ryan Blackburn, Brett H. Mueller II, DO, PhD, and Aparna Ramasubramanian, MD
Edited by Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Autologous and allogeneic hematopoietic stem cell transplantation (HSCT) is an important treatment option for a variety of malignancies, including leukemias and lymphomas. In the United States, more than 20,000 HSCT procedures are performed annually; of these, approximately 12,000 are autologous and 8,000 are allogeneic, and the number of HSC transplants continues to rise annually.1 Despite improvements in immunosuppressive therapies and better human leukocyte antigen (HLA) typing, graft-vs.-host disease (GVHD) remains a common complication, occurring in 30% to 70% of patients.2
 |
|
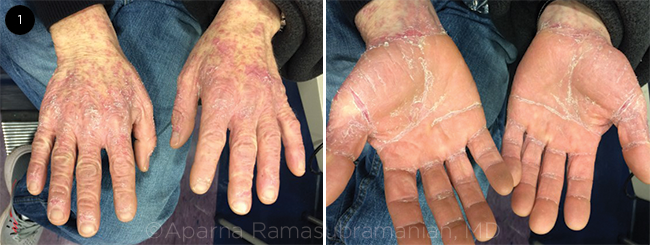
SKIN AT MANIFESTATION. (1) Hands of patient with acute GVHD.
|
Types of GVHD
GVHD is mediated by the donor CD4+ and CD8+ T cells that identify the recipient’s own histocompatibility antigens as foreign and mount an immunological reaction against them.3 Acute GVHD can occur within 100 days of HSCT. Although this disease can affect any part of the human body, the most important clinical consequences involve the skin, liver, intestines, and eyes.3 Acute GVHD can manifest as a skin rash that may advance to generalized desquamation (Fig. 1). The disease can cause the destruction of the liver bile ducts leading to jaundice and liver failure. Ulcerations of the gut mucosa may also occur, resulting in bloody diarrhea.3
Chronic GVHD occurs 100 or more days after HSCT. It may be a continuation of acute GVHD or can occur insidiously.3 It can cause the destruction of skin appendages, fibrosis of the dermis, chronic liver disease, esophageal strictures, and dysfunction of the immune system through the infiltration and fibrosis of the thymus.3 Ultimately, many of these patients succumb to severe recurrent infections.3
Most GVHD is caused by allogeneic bone marrow transplants; however, even patients who have received an autologous or syngeneic (i.e., from an identical twin) bone marrow transplant may occasionally develop an acute GVHD-like syndrome that is described as auto-aggression or engraftment syndrome.4,5 This auto-aggression syndrome has been reported to occur in 8% of patients who received an autologous HSCT.6 Typically, this disease process is self-limited and treatment is not required.
Ocular manifestations have been reported to occur in 40% to 60% of GVHD patients.7 Typical disease presentation can range from meibomian gland obstruction, scarring of the lacrimal gland, and severe dry eye to chronic inflammation leading to conjunctival necrosis, corneal erosions, thinning, ulcerations, and perforations.
It is important for clinicians to keep ocular GVHD in mind when treating patients who have had a bone marrow transplant. The ocular disease may be overlooked, particularly if the patient has severe or life-threatening systemic conditions; and a delay in diagnosis and treatment can lead to increased morbidity due to corneal scarring and decreased vision. If the ocular disease is recognized early, patients are more likely to respond to conservative treatment modalities. The aim of this article is to provide a stepwise approach that can aid in the diagnosis and management of ocular GVHD.
 |
|
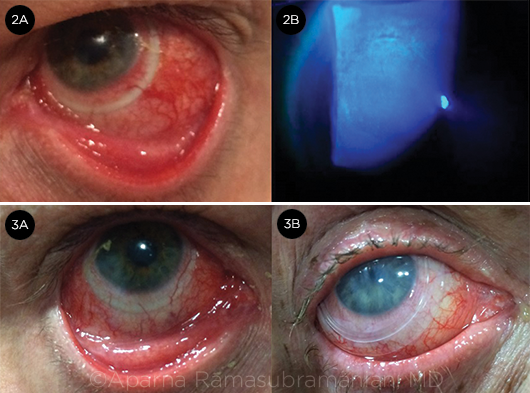
ACUTE OCULAR GVHD. (2A) Conjunctival hyperemia. (2B) diffuse corneal epithelial keratitis. SURFACE PROTECTION. (3A) Scleral contact lens. (3B) Prokera for treatment of refractory epithelial defect.
|
Clinical Diagnosis
Ocular GVHD can present with a wide array of signs and symptoms affecting all layers of the eye, including the eyelids, lacrimal gland, conjunctiva, cornea, vitreous, and choroid; however, the ocular surface and lacrimal gland tend to be most affected.8 Although the manifestations of ocular GVHD are similar to those of other immune-modulated diseases, a history of HSCT, in combination with the following exam findings, helps to establish the clinical diagnosis.
Conjunctiva. Hyperemia is very common and can be associated with pseudomembrane or membrane formation (Fig. 2A). Patients may also present with conjunctival necrosis, cicatricial scarring, and fibrosis. See Table 1 for a proposed grading system for chronic ocular GVHD based upon conjunctival findings.9
Lacrimal gland. T-cell mediated–cytotoxic effects result in fibrosis, which, in turn, leads to irreversible dry eye.
Eyelid. Meibomian gland dysfunction, symblepharon, and trichiasis are common and lead to dry eye and ocular surface damage.
Cornea. Common findings include superior limbic keratoconjunctivitis; limbal stem cell deficiency; filamentary keratitis; corneal neovascularization; and corneal ulceration, melting, and perforation (Fig. 2B).
Lens. Cataract formation is very common in GVHD patients and can be related to corticosteroid treatment or total body irradiation administered in preparation for HSCT.
Posterior segment. Exam findings may include microvascular retinopathy, central serous retinopathy, and posterior scleritis.
Table 1. Conjunctival Grading Criteria for Chronic GVHD
| Grade |
Manifestation |
| 1 |
Conjunctival hyperemia of bulbar / palpebral conjunctiva |
| 2 |
Fibrovascular change of palpebral conjunctiva < 25% of surface of at least 1 eyelid |
| 3 |
Fibrovascular change of palpebral conjunctiva = 25%-75% of surface of at least 1 eyelid |
| 4 |
Fibrovascular change of palpebral conjunctiva > 75% with or without cicatricial entropion of 1 eyelid |
Source: Kim SK. Ocular graft versus host disease. In: Krachmer JH et al., eds. Cornea. St. Louis, Mo: Elsevier Mosby; 2004:879-885.
NIH Diagnostic and Distinctive Signs and Symptoms
The current National Institutes of Health (NIH) consensus criteria separate diagnostic signs and symptoms from distinctive signs and symptoms associated with chronic GVHD. The diagnostic criteria alone are sufficient to determine the presence of chronic GVHD. In contrast, distinctive signs and symptoms indicate possible chronic GVHD, but additional testing is required for confirmation. Such testing may include a confirmatory biopsy, other relevant tests (e.g., Schirmer), or additional clinical evaluation by an ophthalmologist.10 (See also Table 2.)
Diagnostic ocular criteria. A diagnosis of chronic ocular GVHD can be made by one of the following:
- New ocular sicca documented by low Schirmer test, with a mean value of ≤5 mm at 5 minutes not due to other causes
- New onset of keratoconjunctivitis sicca by slit-lamp exam with mean Schirmer test values of 6 to 10 mm not due to other causes.10
Distinctive ocular criteria requiring further investigation include:
- New-onset dry, gritty, or painful eyes
- Cicatricial conjunctivitis
- Keratoconjunctivitis sicca
- Confluent areas of punctate keratopathy
- Photophobia
- Periorbital hyperpigmentation
- Blepharitis
Table 2. Eye Portion of NIH Organ Scoring Matrix for Chronic GVHD*
| Score 0 |
Score 1 |
Score 2 |
Score 3 |
| No Symptoms |
Mild dry eye symptoms not affecting activities of daily living (ADL) (requiring lubricant eye drops ≤3×/day) |
Moderate dry eye symptoms partially affecting ADL (requiring lubricant eye drops >3×/day or punctal plugs), without new vision impairment due to KCS |
Severe dry eye symptoms significantly affecting ADL (special eyewear to relieve pain) or unable to work because of ocular symptoms or loss of vision due to KCS |
*Score is based exclusively on dry eye severity.
Source: Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e381.
Management
Ophthalmologic examination to monitor for ocular GVHD is recommended every 3 to 12 months for all patients who have received HSCT and who are currently on systemic immunosuppressive therapy. The median duration of treatment is generally 2 to 3 years.
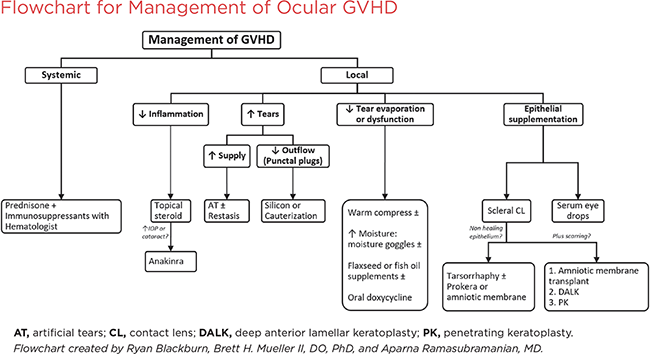
The basic principles of treatment for ocular GVHD include a stepwise approach to decrease ocular inflammation, increase lubrication, prevent evaporation of the tear film, and protect the corneal epithelium. (See also the management flowchart below.)
Systemic treatment. Management of ocular GVHD begins with systemic treatment, usually tailored to the individual patient by the hematologist/oncologist, and may include corticosteroids in combination with other immunosuppressant therapies. These treatments should improve ocular manifestations when combined with local measures.
Local treatment. The NIH chronic GVHD consensus workshop recently updated recommendations on the management of chronic GVHD, including ocular GVHD. The group outlined 4 main supportive goals for the treatment of ocular GVHD: lubrication, decrease in ocular inflammation, control of drainage, and control of evaporation.11 Our recommendations for meeting these goals include the following therapeutic measures.
I. Increase Lubrication
A. Preservative-free artificial tears. This recommendation received the only A level rating (i.e., should be offered to all patients) for ocular GVHD.11 Different brands may need to be tried to maximize benefit.
B. Viscous ointment. To be used at bedtime.
C. Hydroxypropyl methylcellulose pellets (Lacrisert). May be helpful for patients who use artificial tears often.
D. Oral cevimeline or pilocarpine. Has been shown to improve sicca symptoms in Sjögren syndrome. Drug interactions and effects on other disease processes such as angle-closure glaucoma must be reviewed.
E. N-Acetylcysteine (mucolytic). If multiple corneal filaments are present.
II. Decrease OcularInflammation
A. Topical steroids.Valuable, but should be continued only for a limited time as needed during acute ocular GVHD exacerbation. Can be used while systemic steroids are being tapered. Chronic use of topical steroids can lead to infection, cataracts, and increased IOP.
B. Topical cyclosporine (Restasis). Reduces immune response on the ocular surface and may improve tear function in ocular GVHD patients.
C. Lifitegrast ophthalmic solution (Xiidra). Downregulates T-cell–mediated inflammatory reactions.
D. Autologous serum eyedrops. Can be started if there is progression despite use of methods above. Contains growth factors and vitamins that support the health of the ocular surface. However, because the drops must be compounded from each patient’s serum, the availability of this treatment may be limited.
E. Topical anakinra 2.5%. Interleukin 1 receptor antagonist now being studied to reduce inflammation and treat dry eye disease. Has shown good results in initial trials and case reports.12
III. Treat Meibomian Gland Disease (MGD)
A. Lid hygiene. Helps to clear blockages and restore normal output of the meibomian glands. Easy and inexpensive but requires daily adherence.
1. Removing eye makeup before bedtime helps prevent the gland openings from getting plugged. Also performing periodic lid scrubs can help remove debris from the gland openings.
2. Warm compresses are an easy and inexpensive way to help to clear blockages and restore normal output of the meibomian glands; daily adherence is required for full benefit.
B. Topical antibiotics (e.g., azithromycin). Can help reduce meibomian gland blockage if lid hygiene/compresses are not sufficient; exact mechanism is unknown.
C. Fish oil or flaxseed oil (omega-3) dietary supplementation. Helps to improve the quality and consistency of the meibomian gland secretions.
D. Oral doxycycline or minocycline. These drugs have anti-inflammatory and antibiotic effects. May be used if MGD is severe and there are no systemic contraindications. Coordinate use of doxycycline with patient’s hematologist/oncologist to avoid potential drug interactions.
IV. Provide Surface Protection
A. Scleral contactlenses (e.g., PROSE). Provide symptomatic relief with improved vision and comfort in patients with epithelial damage (Fig. 3A). Help to protect the cornea, maintain fluid on the cornea, promote healing, and reduce evaporation.
B. Amniotic membrane devices
1. Prokera ocular bandage device. Consists of amniotic membrane tissue stretched over a ring, which is inserted like a contact lens (Fig. 3B) without sutures.
2. Ambiodisk: Amniotic membrane disk; may be held in place with sutures, tissue adhesive, or contact lens.
V. Consider Surgical Options
A. Tarsorrhaphy. Protects the cornea and decreases dryness/evaporation if significant corneal ulceration is present.
B. Punctal occlusion. Decreases tear drainage. Consider use in all chronic GVHD patients; thermal cauterization may be necessary if plugs are frequently dislodged.
C. Multilayeramniotic membrane transplantation. May be used as the treatment for severe dry eyes and calcareous corneal degeneration with or without perforation; also helps to prevent symblepharon formation.
D. Lysis of membranes and symblepharon. Helps prevent lid ectropion and lagophthalmos and subsequent conjunctival scarring.
E. Deep anterior lamellar keratoplasty or penetrating keratoplasty. May be performed in very advanced cases of ocular GVHD if there is descemetocele formation or corneal perforation.
___________________________
1 Pasquini M, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2016. www.cibmtr.org. Accessed March 19, 2017.
2 Antin JH. N Engl J Med. 2002;347(1):36-42.
3 Kumar V et al. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia: Elsevier Saunders; 2014.
4 Cornell RF et al. Blood Marrow Transplant. 2015;21(12):2061-2068.
5 Batra A et al. Bone Marrow Res. 2014:891427.
6 Hood AF et al. Arch Dermatol. 1987;123(6):745-750.
7 Flowers ME et al. Blood. 2002;100(2):415-419.
8 Kim SK. Ocular graft versus host disease. In: Krachmer JH et al., eds. Cornea. St. Louis, Mo: Elsevier Mosby; 2004:879-885.
9 Robinson MR et al. Bone Marrow Transplant. 2004;33(10):1031-1035.
10 Jagasia MH et al; National Institutes of Health Consensus Development Project on Criteria for
Clinical Trials in Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015;21(3):389-401.e381.
11 Carpenter PA et al.; National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015(7):1167-1187.
12 Amparo F et al. JAMA Ophthalmol. 2013;131(6):715-723.
___________________________
Mr. Blackburn is a medical student, Dr. Mueller is an ophthalmology resident, and Dr. Ramasubramanian is an assistant professor of ophthalmology; all are at the University of Louisville, Louisville, Ky. Relevant financial disclosures: None. This work was supported in part by an unrestricted institutional grant from Research to Prevent Blindness.
(click to expand)