By Kenneth C. Mathys, MD
Edited by Thomas A. Oetting, MD
This article is from November 2007 and may contain outdated material.
After a two-week honeymoon in Virginia, returning to work was a shock to the system for Mary Sanchez,* a 31-year-old Hispanic female. She spent much of the first two days fielding inquiries about her vacation and working her way through a backlog of voice- and e-mails. On the third day, just as she was starting to get a handle on things, she started to suffer blurry vision associated with headache, photophobia, red eyes and bilateral eye pain. She left work early and, since it was a Friday, she hoped that a weekend of rest would do the trick. But by the next day, her vision had gotten worse and she decided to see a doctor.
The Patient’s Medical History
Her past medical history was significant for bipolar disorder, for which she had been taking lamotrigine, quetiapine and clonazepam for many months. She also had migraine headaches for which she was taking acetaminophen with oxycodone, as needed. She had no known drug allergies. The review of systems was negative for chest pain, shortness of breath, dizziness, loss of consciousness and tinnitus. She smoked one pack of cigarettes per day but denied any alcohol or illicit drug use.
She denied diplopia, photopsias or transient visual obscurations. She did not wear corrective lenses, and there was no history of ocular trauma. In short, Ms. Sanchez had no prior ophthalmic history, although her family ocular history was significant for glaucoma in her grandfather.
The Patient’s Physical Exam
Ms. Sanchez was alert and oriented, and her vital signs were stable. Cardiovascular and pulmonary exams were normal. The neurologic exam was nonfocal. Her uncorrected visual acuity was count fingers at three feet in both eyes, and her best-corrected visual acuity was 20/60 in the right eye and 20/30–2 in the left with a manifest refraction of –5.25 D. She had an IOP of 48 mmHg in both eyes by applanation tonometry.
There was no anisocoria and she had no relative afferent pupillary defect in either eye. Visual fields were constricted to confrontation testing bilaterally. Her motility was normal.
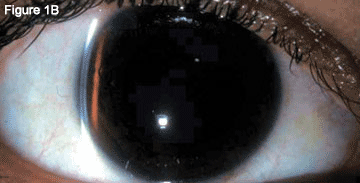
The slit-lamp examination showed bilateral hyperemic conjunctivae. The corneas were clear without edema or abrasion. The anterior chambers were very shallow centrally and peripherally (Fig. 1A). Her lenses were clear.
Gonioscopy showed 360-degree angle closure bilaterally. The dilated fundus examination revealed cup-to-disc ratio of 0.5 in both eyes.
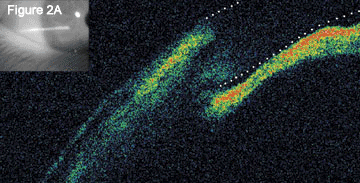
Optical coherence tomography of the angles showed angle closure with anterior displacement of the irides (Fig. 2A).
|

|
|
Before and After Treatment. When we examined the patient, we discovered conjunctival hyperemia. Her anterior chambers were initially very shallow (1A) but, after treatment, these deepened (1B).
|
Early Misdiagnosis
An initial diagnosis of acute angle-closure glaucoma was made and therapy was instituted to lower her IOP with topical (dorzolamide/timolol, brimonidine and pilocarpine ophthalmic drops) and oral (500 mg acetazolamide) medications.
Two hours later, the IOP was unchanged, and so a YAG peripheral iridotomy was performed in each eye. The postprocedure pressures were unchanged. The patient was given another 500 mg of acetazolamide and a 1-mg/kg dose of oral glycerine. The brimonidine and pilocarpine drops were repeated.
After another two hours, the IOP was 38 mmHg in both eyes. The patient was sent to the emergency department and received a 2-mg/kg intravenous infusion of mannitol.
One hour after the infusion, her IOP was 28 mmHg in both eyes. The patient was discharged home on topical pilocarpine 1 percent, dorzolamide/timolol drops and oral acetazolamide. Because of the possibility of angle closure secondary to a medication reaction, the lamotrigine, quetiapine and clonazepam were held.
The next morning, her vision had still not improved. Indeed, her photophobia and eye pain had worsened. Her best-corrected visual acuity was 20/70 in both eyes with a manifest refraction of –5.75 D in the right eye and –6.25 D in the left. The IOP was 20mmHg in both eyes. The conjunctivae remained hyperemic and the anterior chamber remained shallow with moderate anterior chamber reaction consisting of both pigmented and nonpigmented cells.
We found a patent peripheral iridotomy in the right eye, but the peripheral iridotomy in the left eye was not patent. The axial length was 23 mm. B-scan ultrasonography showed attached retinas and choroidal thickening bilaterally.
|
|
|
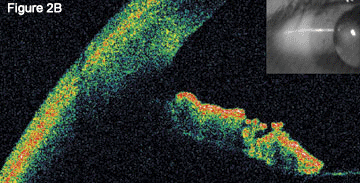
Optical Coherence Tomography. When we initially performed OCT on Ms. Sanchez, the images showed angle closure (2A); this image has been enhanced to define the boundary of the anterior and posterior cornea). When we repeated OCT after treatment, we found that the normal anterior chamber anatomy had returned (2B).
|
Diagnosis Reconsidered
The patient had not responded as predicted to typical therapy for acute angle-closure glaucoma.
Differential diagnosis included bilateral angle-closure glaucoma, bilateral aqueous misdirection syndrome (malignant glaucoma), as well as a reaction to a systemic medication causing choroidal effusion with secondary angle closure. A literature review for such a reaction attributable to the patient’s current medications was unrevealing.
Malignant glaucoma was unlikely as the patient had no prior ocular surgery. Despite no obvious cause, the presentation was most consistent with a systemic medication reaction causing choroidal effusion and secondary angle closure.
Treatment and New Diagnosis
The pilocarpine was discontinued and cycloplegic therapy was instituted using cyclopentolate ophthalmic drops every eight hours. Dorzolamide/timolol and acetazolamide were continued, and she was given prednisolone acetate 1 percent ophthalmic drops every hour in both eyes.
The next day her pain and photophobia were significantly improved and the visual acuity had subjectively improved.
Two days later, her eye pain had resolved. Best-corrected vision was 20/40 in the right eye and 20/30 in the left. The myopic shift had resolved with a new manifest refraction of +0.5 D in each eye. Her IOP was 11 mmHg in the right eye and 12 mmHg in the left. The anterior chambers were deep and had moderate cell (Fig. 1B). Gonioscopy showed an open iridocorneal angle 360 degrees with D-40r 2+ configuration in both eyes.
Repeat optical coherence tomography showed normal angle architecture (Fig. 2B).
The patient was maintained on phenylephrine 2.5 percent and scopolamine 0.25 percent drops for many months. Her oral and topical IOP-lowering medications were all stopped. She became pregnant and the drops were held. She had no recurrence of signs or symptoms of angle closure.
On follow-up questioning the patient admitted to taking topiramate for three days one month prior to the onset of her symptoms. She was using the medication for treatment of her migraine headaches. Topiramate is known to cause choroidal effusion and acute secondary angle-closure glaucoma similar to the case previously described. Diagnosis of topiramate-associated, acute secondary angle-closure glaucoma was confirmed.
About the Disease
Topiramate is an antiepileptic drug that is also used for migraine, seizure and neuropathic pain.
Its use has been associated with many cases of acute, bilateral secondary angle-closure glaucoma.1-7 This classically presents with bilateral angle closure, ocular hypertension, bilateral acute myopic shift and shallow central and peripheral anterior chambers. Suprachoroidal effusions and ciliary body edema are often documented. The ocular hypertension does not respond to therapy for acute angle-closure glaucoma, including peripheral iridotomy and miosis. Definitive treatment consists of cycloplegia.
Topiramate is a sulfamate-substituted monosaccharide. Other sulfa-based drugs including acetazolamide, hydrochlorothiazide and cotrimoxazole have also been associated with secondary angle-closure glaucoma.8
Quigley eloquently describes the mechanism of topiramate-associated acute secondary angle closure. He posits that a developing choroidal effusion will exert pressure on the vitreous body, possibly resulting in resistance to flow through the vitreous body. The vitreous then compresses the lens-iris diaphragm, displacing it anteriorly. Fluid passing through the vitreous can only gain access to the anterior chamber through the area between the vitreous base and the central adherence of the anterior hyaloid face to the posterior lens. Cycloplegia increases the diffusional area for fluid to leave the vitreous cavity by widening the ciliary body diameter.9
This case is unusual as the patient had not taken topiramate for one month prior to onset of symptoms. Eighty-five percent of cases of topiramate-associated angle-closure glaucoma develop within the first two weeks of treatment, but reactions have been reported within hours of a first dose or as long as seven weeks after the onset of therapy. The onset of symptoms occurs a mean of seven days after starting topiramate.10It is critical to inquire about current and past medication use when evaluating patients with acute angle-closure glaucoma.
___________________________
* Patient name is fictitious.
___________________________
Dr. Mathys is in his final year of residency at the University of North Carolina, Chapel Hill.
___________________________
1 Fraunfelder, F. W. et al. Ophthalmology 2004;111:109–111.
2 Banta. J. T. et al. Am J Ophthalmol 2001;132:112–114.
3 Rhee, D. J. et al. Arch Ophthalmol 2001;119:1721–1723.
4 Sankar, P. S. et al. Arch Ophthalmol 2001;119:1210–1211.
5 Craig, J. E. et al. Am J Ophthalmol 2004;137:193–195.
6 Chen, T. C. et al. Br J Ophthalmol 2003;87:648–649
7 Medeiros, F. A. et al. Arch Ophthalmol 2003;121:282–285.
8 Lachkar, Y. et al. Curr Opin Ophthalmol 2007;18:129–133.
9 Quigley, H. A. et al. J Glaucoma 2003;12:167–180.
10 Fraunfelder, F. W. et al. Ophthalmology 2004;111:1275–1279.