Treating ROP in China: Ranibizumab Proves Effective
By Lynda Seminara and selected by Stephen D. McLeod, MD
Journal Highlights
Ophthalmology, August 2017
Download PDF
To determine anatomic outcomes and influential factors of ranibizumab in the treatment of retinopathy of prematurity (ROP), Huang et al. reviewed data for infants with ROP in China who received intravitreal injections of the drug as primary therapy. They found that ranibizumab appeared to be effective in the treatment of these patients. In addition, the results indicated that patient age and ROP type may help predict the risk of disease reactivation.
For this retrospective study, the researchers included 145 patients (283 eyes) with type 1 ROP who received a single injection of ranibizumab in each eye (0.25 mg/0.025 mL, or half the adult dose) as primary treatment. Fundus photography was performed when the infants were initially screened for ROP and throughout follow-up, which continued for at least 6 months after the intravitreal injection.
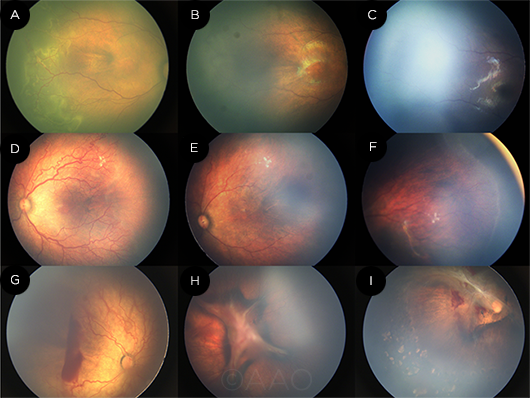 |
RESPONSE. In the regression without reactivation subgroup, this patient had zone II stage 2+ disease (A) that regressed after 1 week of treatment with intravitreal ranibizumab (B). After 40 weeks of treatment, the ROP had regressed with vascularization of zone III (C). A patient in the regression with reactivation subgroup was treated for aggressive posterior ROP (D) and experienced regression after 1 week of treatment (E). However, 9 weeks later, the disease had reactivated (F). A patient in the negative/no response subgroup was treated for aggressive posterior ROP (G). Following treatment, the posterior fibrosis worsened and the disease progressed to stage 4b (H). The patient was then treated with laser and vitrectomy; at the latest follow-up, the fibrous membrane traction was significantly released (I).
|
A favorable response to ranibizumab was observed in 266 eyes (94%); in contrast, no response or worsening of disease was noted for 17 eyes (6%). Of those infants who responded favorably, 139 eyes had disease regression that was maintained throughout follow-up, and 127 eyes had regression with subsequent reactivation of disease. Vascular abnormalities or incomplete vascularization was noted in 8 eyes, despite absence of reactivation. At the last follow-up examination, the retina was attached in 278 eyes and detached in 5 eyes. Complications included cataract (2 eyes) and vitreous preretinal hemorrhage (1 eye). Overall, 152 eyes (54%) had laser or surgical treatment after intravitreal ranibizumab.
Results of classification tree modeling indicated that the likelihood of disease reactivation was 62% if ranibizumab was given at a gestational age (GA) of ≤ 29.5 weeks and only 30% if performed later. However, patients who received the drug at GA ≤ 29.5 weeks who also had zone II stage 2+ ROP had a relatively low incidence of reactivation (38% vs. 80% for other patients in this GA group).
The original article can be found here.