Download PDF
Second only to diabetic retinopathy as a leading cause of retinal vascular blindness, retinal vein occlusion (RVO) is often linked with systemic risk factors such as hypertension, arteriosclerosis, diabetes, and smoking and with other ocular diseases such as glaucoma.
Although RVO most often occurs after age 65, it is also seen in younger patients in the setting of hypercoagulable states such as Factor V Leiden mutations or systemic inflammatory diseases such as lupus. In these younger patients, a systemic workup is critical, said Sharon Fekrat, MD, at the Duke Retinal Vein Occlusion Center of Excellence in Durham, N.C.
RVOs occur only in humans, and there are few animal models of the disease process, said Peter A. Campochiaro, MD, at the Wilmer Eye Institute. “Most of what we know about its pathogenesis we've learned by following patients.
Five retina experts share what they've learned from their clinical experience and from major RVO studies in recent years.
Comprehending a Complex Disease
RVOs cause an increase in venous pressure, with subsequent massive edema and hemorrhages throughout the affected area, said Dr. Campochiaro. These changes are accompanied by retinal ischemia that ranges from mild in some patients to very severe in others, he said.
In ischemic retina, there are increased levels of the products of hypoxia regulated genes, including vascular endothelial growth factor (VEGF). The development of specific VEGF antagonists allowed studies showing that VEGF plays a critical role in macular edema, hemorrhages, and worsening of retinal ischemia,” Dr. Campochiaro said. Macular edema is a treatable cause of vision loss in these eyes, said Dr. Fekrat, whereas a lack of perfusion (ischemia) due to capillary nonperfusion can contribute to irreversible vision loss.
Types of RVO
The main difference among the types of retinal vein occlusions is their location, said Seenu M. Hariprasad, MD, at the University of Chicago. Central retinal vein occlusions (CRVOs), which occur in roughly 20 to 30 percent of patients with RVO, are located inside or at the head of the optic nerve. “About 3 percent of RVOs, hemiretinal vein occlusions [HRVOs] land at the sweet spot, right as the vessel separates from the nerve,” he said. Located more distally, branch retinal vein occlusions (BRVOs) occur in 60 to 70 per cent of patients with this condition.
CRVO. The etiology of CRVO is not clearly understood, so current treatments address its sequelae, said Phoebe Lin, MD, PhD, at Oregon Health & Science University in Portland. It is thought that a thrombus occurs at the central retinal vein, proximal to the lamina cribrosa, but attempts to lyse the obstruction with tissue plasminogen activator have met with mixed results. The variation is likely related to the timing of when the patient presents in the clinic after the primary event, she said.
Another perplexing aspect is that people on an anticoagulation regimen aren't immune to these vein occlusions, which is inconsistent with the prevailing theory of clots, said Colin A. McCannel, MD, at Jules Stein Eye Institute in Los Angeles. After many years of study, the pathogenesis of CRVO is still a bit of a black box, and in that box may be some treatment targets that we currently don t know about.”
BRVO. In contrast, “We do think we have a pretty good handle on the patho genesis of BRVO,” said Dr. McCannel. It occurs at areas where the arteries and veins cross, sharing a common connective tissue. The artery walls thicken, compressing the vein and precipitating production of a clot.” The overall prognosis for BRVO is better than for CRVO, with visual outcomes depending upon the density of the occlusion, he said. However, when BRVO is more ischemic, and the ischemia involves the fovea, vision tends to improve very little, if at all.
HRVO. There is no consensus about whether HRVO is a variant of BRVO or CRVO or is a separate type altogether, said Dr. McCannel.
Treatment Options
Regardless of the classification, venous occlusive disease tends to respond well to antiangiogenic therapy, which many experts now consider the first-line approach. Corticosteroids, as well, may be appropriate for selected patients. In addition, the availability of ultra-widefield fluorescein angiography (FA) imaging is reviving interest in laser treatment.
Lasers Yesterday and Today
Long before the anti-VEGF era, there was controversy about the use of laser for RVO, said Dr. Hariprasad. “In the 1980s and 1990s, the Branch Vein Occlusion Study (BVOS) and Central Vein Occlusion Study (CVOS) became the first prospective, multicenter, randomized studies on this disease.” Although the numbers were small, the BVOS trial established macular grid laser as the nearly 20-year gold standard for treating macular edema due to BRVO, while the CVOS trial failed to show clear visual benefits of macular laser for CRVO.1,2
Current trends in laser use. Today, laser therapy may still play a role for BRVO patients, said Dr. Campochiaro, particularly those requiring many anti-VEGF injections. The need for ongoing injections may be attributable to a VEGF drive coming from the ischemic peripheral retina, said Dr. Hariprasad, and with widefield FA, the clinician can now view previously elusive areas of peripheral nonperfusion.
With this new imaging technology, said Dr. McCannel, there appears to be a trend among retina specialists to treat the nonperfused retina with scatter laser in an attempt to reduce the VEGF burden in the eye and the need for anti-VEGF therapies. “While this is not a proven approach and not supported by any clinical trials, anecdotally it is becoming more commonplace,” said Dr. McCannel. “We do not yet fully understand the role of directed scatter photocoagulation in the long-term management of RVOs, but it appears to address part of the pathophysiology of macular edema.” Dr. Campochiaro is currently conducting a trial to learn more about this approach.
Dr. Lin also finds laser useful in some cases: “If patients have cystoid macular edema and peripheral capillary nonperfusion, I will consider targeted photocoagulation guided by ultra-widefield FA to see if that decreases their CME or the need for injections.”
5 Pearls for Practice
Dr. Campochiaro: Macular edema is not our only endpoint. Even when edema abates and vision is stable, nonperfusion can progress over time. Therefore, we need to occasionally check a patient’s vasculature. Widefield FA is particularly helpful in revealing the degree of nonperfusion.
Dr. Fekrat: A patient who has RVO in one eye will often ask, “What can I do to prevent a CRVO in my other eye?” This is a prime opportunity to discuss management of associated systemic conditions, such as hypertension, and adoption of a healthier lifestyle, including improved nutrition and daily exercise.
Dr. Hariprasad: If a patient has had eight anti-VEGF injections and still has recalcitrant fluid, why do you think the ninth one will make any difference? Consider the Ozurdex implant. On the flip side, if you started a patient on Ozurdex and fluid persists, why not try an anti-VEGF agent? Think about combination therapy; there is good evidence that both inflammation and high VEGF levels are implicated in this disease.
Dr. Lin: If anti-VEGF agents don’t seem to work or the CME becomes refractory to anti-VEGF, I try macular grid laser and an intravitreal steroid injection or consider targeted retinal photocoagulation.
Dr. McCannel: When a patient requires bilateral injections, I never use the same lot number for both eyes. This reduces the risk of a catastrophic outcome in the unlikely event that a medication is contaminated. |
Intravitreal Steroids
“The steroid era was kicked off with the SCORE studies in 2009,” said Dr. Hariprasad, “providing clear guidance about the use of steroids and laser in this disease. Soon after, Allergan’s GENEVA study resulted in Ozurdex becoming the first FDA-approved treatment for macular edema secondary to RVO.”3
Although the reasons for the efficacy of steroids in CME related to RVO are not fully understood, said Dr. Lin, you can think of them as working upstream of VEGF by suppressing its expression. “However, it’s not possible to know ahead of time how much the inflammatory component is driving the condition in a particular patient,” she said.
Triamcinolone acetonide. In 2009, the Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE )study compared two doses of preservative-free intravitreal triamcinolone acetonide (IVTA; Trivaris) for the treatment of CME in eyes with CRVO. “This was the first big breakthrough for CRVO,” said Dr. Lin. “Steroids in the SCORE trial provided improvement in visual acuity and reduction of vision loss compared with the observation group.” In BRVO patients, SCORE found that triamcinolone and laser achieved similar visual outcomes.4
Today, Dr. Fekrat usually reserves IVTA (Triesence) for patients without glaucoma who are pseudophakic or cannot tolerate repeated anti-VEGF injections.
Dexamethasone intravitreal implant (Ozurdex). This biodegradable injectable implant delivers dexamethasone to the back of the eye. Far less costly than anti-VEGF injections—but more expensive than triamcinolone—the implant slowly dissolves in the eye over the course of three to five months, said Dr. Hariprasad, who noted that he has never injected more than three Ozurdex implants in an eye in any given year. “Although its efficacy is not as good as that of anti-VEGF therapy, there is a population of ‘one-hit wonders’ who only need one Ozurdex injection to completely resolve their macular edema.”
Dr. Lin reserves Ozurdex for patients who are refractory to anti-VEGF therapy, patients who have had vitrectomy and no longer have a vitreous gel to sustain the release of an intravitreal injection, or patients who have had a good response to triamcinolone in the past but experienced elevated intraocular pressure (IOP). Owing to the risk of anterior migration of the implant, she said, Ozurdex should be used with caution in vitrectomized patients with an open or absent posterior capsule.
Dr. McCannel agreed with that concern: “One should think three times before putting an Ozurdex implant into aphakic or pseudophakic eyes with an open posterior capsule. Implants can be swept into the anterior chamber by the aqueous fluid dynamics inside the eye.”
According to Dr. Campochiaro, results with Ozurdex are good, but you have to inject the implant more frequently than originally expected. “While the initial studies showed few problems because injections were done every six months, in many patients, injections are needed every three to four months,” he said. “Cataract progression and increased IOP are not uncommon with more frequent injections.”
Whether triamcinolone or Ozurdex, steroids ultimately promote cataracts, said Dr. Fekrat, adding that the side effect profiles of steroids and anti-VEGF therapy aren’t close to comparable. Despite “recent rumblings” about elevated IOP with long-term anti-VEGF use, she said, pressure appears to be well controlled with medication.
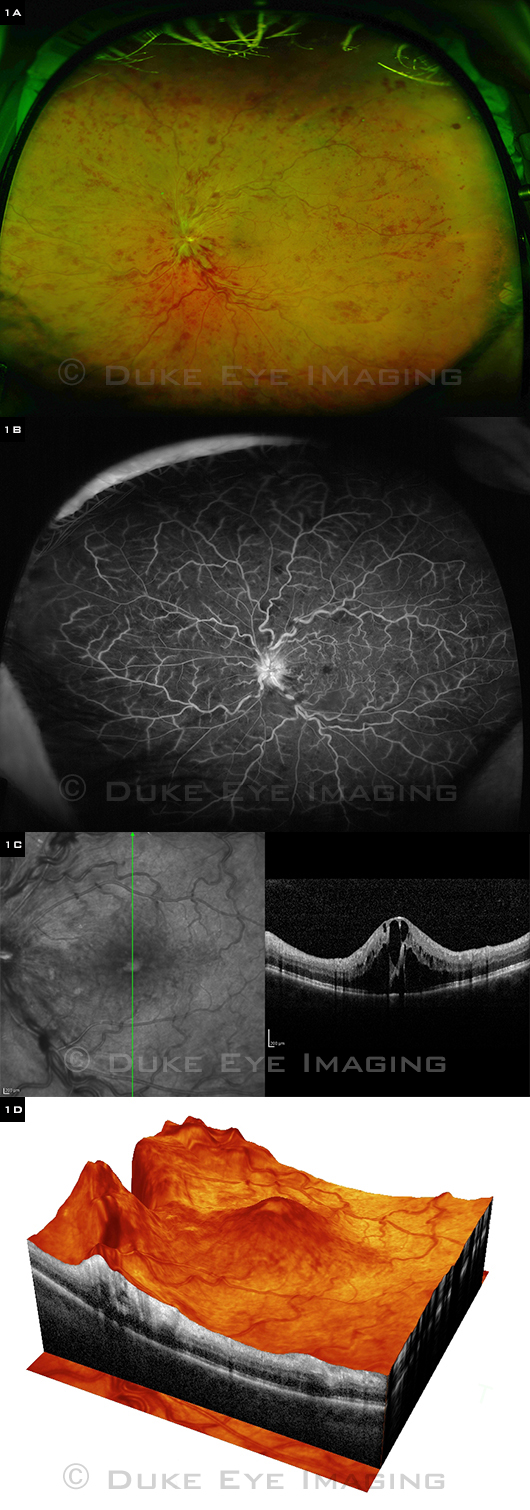 |
|
PERFUSED CRVO. Left eye of adult patient shows marked cystoid macular edema. (1A) Ultra-widefield color fundus photo. (1B) Ultra-widefield FA is ideal for evaluating peripheral retinal capillary perfusion. (1C) Vertical SD-OCT image. (1D) 3-D rendering of SD-OCT images.
|
Anti-VEGF Agents
Suppressing VEGF with specific antagonists markedly reduces the macular edema of RVO, said Dr. Campochiaro. “The improvement seen in the vein occlusion trials is better than that seen in any other disease process, suggesting that VEGF is particularly important in this disease.” Even with substantial nonperfusion, there can still be unanticipated, yet significant, visual improvement with antiangiogenic therapy, added Dr. McCannel.
Ranibizumab (Lucentis). From the results of the BRAVO and CRUISE studies completed in 2009, said Dr. Lin, we have learned that anti-VEGF therapy works well to restore vision loss due to macular edema associated with either branch or central retinal occlusion.5,6 Ranibizumab is now approved for both indications.
The CRUISE trial, which looked at ranibizumab versus sham observation, offered a particularly big step up for CRVO patients, she said. “We went from having no intervention, or steroid injections that caused cataract and elevated intraocular pressure, to actually improving visual acuity by three lines in almost 50 percent of patients without [those] side effects.”
Aflibercept (Eylea). Regeneron recently conducted similar studies: GALILEO in Europe and COPERNICUS in the United States. About 55 percent of subjects had improvement of three lines or more, said Dr. McCannel. “This provided confirmation of what was found in the Lucentis studies. Overall, the results were strikingly similar.”
Bevacizumab (Avastin). There are no robust data for Avastin—only small case series and retrospectives—and they also seem to have similar treatment results, said Dr. McCannel. “I’d venture to say that Lucentis, Avastin, and Eylea all perform similarly.”
Choosing among anti-VEGF drugs. With no prospective, head-to-head clinical trials comparing the three, which drug is best remains an open question, said Dr. Lin.
- Cheaper. Avastin is used off label for RVO but, at about $35 to $40 a dose, is less expensive than on-label therapies, said Dr. Hariprasad. This may provide a financial incentive for younger RVO patients who have commercial insurance with high deductibles or copays, said Dr. Fekrat. However, it is also important to remember that Avastin is a compounded medication, and there are concerns about the potential increased risk of contamination. “Scrutinize the compounding pharmacy you utilize,” she said, “including examining its track record and compounding practices.” The Academy recommends looking for a pharmacy that is accredited by the Pharmacy Compounding Accreditation Board (PCAB) and that adheres to the USP 797 standards.
- Faster clearing. Dr. Campochiaro mainly uses ranibizumab because it clears very quickly once it gets outside the eye, which may lessen systemic risks.
- Greater longevity. Approved for CRVO, Eylea has less of a track record than Lucentis, said Dr. Hariprasad, but potentially has greater pharmacological longevity. “Eylea also binds to placental growth factor,” said Dr. Lin, “but we don’t yet know how strong a role this plays in the etiology of CME due to retinal vein occlusion.”
“I often switch the ‘frequent flyers’ who need monthly injections to Eylea,” said Dr. McCannel. “A large number of my patients who had recurrence at four weeks on Lucentis now end up at exactly six weeks on Eylea.”
Treat aggressively—and early. If you treat aggressively with an anti-VEGF, you can reduce levels of VEGF and the worsening of nonperfusion, said Dr. Campochiaro. “When you treat early enough, you can even cause reperfusion in some areas.”
How long can you wait? “In BRAVO and CRUISE, patients in the sham group, who had a delay in treatment onset of six months, had significantly poorer outcomes than the treatment groups at one year,” said Dr. Campochiaro. “With additional follow-up and treatment in HORIZON, sham BRVO patients on average caught up, but sham CRVO patients did not. Thus, BRVO seems to be a bit more forgiving regarding a short delay in treatment than CRVO.”
Dr. McCannel recommends initiating treatment at the time of diagnosis. “There is no reason to wait,” he said, “as the risk of delay outweighs the risk of treating patients who may resolve spontaneously.”
Duration of treatment. If anti-VEGF therapy is stopped too soon, vessels immediately become nonperfused again, said Dr. Campochiaro. “We still don’t know how long you must treat aggressively to keep those reperfused vessels from closing down.”
A chronic course? “We originally thought there would be resolution of the disease process as collateral vessels developed and bypassed the obstruction, which would eliminate the need for injections,” Dr. Campochiaro continued. “That does seem to occur in some patients, but, surprisingly, the majority of patients continue to require injections for years. They may develop more and more ischemia and higher VEGF levels and therefore become less responsive to treatment.”
A Declining Role for Surgery
Given the availability of good intraocular treatments, the number of patients requiring surgeries such as vitrectomy and membrane peeling has fallen off markedly, said Dr. Hariprasad.
Anti-VEFG changed the picture. In addition to drying the retina, anti-VEGF agents reduce the risk of complications requiring surgery such as neovascularization, vitreous hemorrhage, and tractional retinal detachment, he said. Another consideration to keep in mind, said Dr. Fekrat, is that removing the vitreous gel shortens the half-life of these injectable drugs.
Efficacy not demonstrated. Time has proved that vitrectomy with radial optic neurotomy or arteriovenous sheathotomy are not very robust treatment approaches, said Dr. McCannel. “Similarly, surgery to remove clots from the branch vein occlusions is, at best, highly experimental with little documentation of efficacy.”
Major RVO Studies
SCORE-CRVO: Triamcinolone vs. Observation
Participants: 271 participants with macular edema (ME) associated with CRVO.
Intervention: No therapy or 1 mg or 4 mg of intravitreal triamcinolone every 4 months for 1 year; 2 years of follow-up with a smaller group.
Main outcome measure: Gain of ≥15 letters from baseline to month 12.
Results: Visual gains of 3 or more lines in 27% of 1-mg group, 26% of 4-mg group, and 7% of observation group; higher rates of cataract and increased IOP in the 4-mg group.
Reference: Ip MS et al. Arch Ophthalmol. 2009;127(9):1101-1114.
SCORE-BRVO: Grid Laser vs. Triamcinolone
Participants: 411 participants with ME associated with BRVO.
Intervention: Standard grid laser treatment (average of 1.5 treatments) or intravitreal triamcinolone (average of 2 treatments) for 1 year; 3 years of follow-up with a smaller group.
Main outcome measure: Gain of ≥15 letters from baseline to month 12.
Results: No significant differences among the treatment groups.
Reference: Scott IU et al. Arch Ophthalmol. 2009;127(9):1115-1128.
GENEVA: Ozurdex vs. Sham
Participants: 1,267 participants with ME associated with BRVO or CRVO.
Intervention: Ozurdex 0.7 mg, Ozurdex 0.35 mg, or sham; 6 months of follow-up.
Main outcome measure: Time to achieve a ≥15-letter improvement in best-corrected visual acuity (BCVA).
Results: Time to improvement was significantly less in both Ozurdex groups compared with sham.
Reference: Haller JA et al. Ophthalmology. 2010;117(6):1134-1146.
BRAVO: Ranibizumab vs. Sham
Participants: 397 participants with ME associated with BRVO.
Intervention: Monthly injections of 0.3 mg or 0.5 mg of ranibizumab or sham injections; 6 months of treatment; 6 months of observation.
Main outcome measure: Mean change from baseline BCVA at 6 months.
Results: At 6 months, patients who received 0.3 mg of ranibizumab had a mean gain from baseline of 16.6 letters, and those who received 0.5 mg of ranibizumab had a mean gain of 18.3 letters, compared with 7.3 letters in patients receiving sham injections.
Reference: Campochiaro PA et al. Ophthalmology. 2010;117(6):1102-1112.
CRUISE: Ranibizumab vs. Sham
Participants: 392 participants with ME associated with CRVO.
Intervention: Monthly injections of 0.3 mg or 0.5 mg of ranibizumab or sham injections; 6 months of treatment, 6 months of observation.
Main outcome measure: Mean change from baseline BCVA.
Results: At 6 months, patients in the 0.3- and 0.5-mg ranibizumab treatment groups gained a mean of 12.7 and 14.9 letters, respectively, compared with 0.8 letters in the sham group.
Reference: Brown DM et al. Ophthalmology. 2010;117(6):1124-1133.
COPERNICUS: Aflibercept vs. Sham
Participants: 189 participants with ME secondary to CRVO.
Intervention: Monthly injections of 2 mg of aflibercept or sham injections; 6 months of treatment; 6 months of follow-up.
Main outcome measure: Proportion of eyes with a ≥15-letter gain in BCVA.
Results: 56.1% of aflibercept-treated eyes gained ≥15 letters from baseline compared with 12.3% of sham-treated eyes.
Reference: Boyer D et al. Ophthalmology. 2012;119(5):1024-1032.
GALILEO: Aflibercept vs. Sham
Participants: 177 participants with ME secondary to CRVO.
Intervention: 2 mg of aflibercept or sham injections monthly; 6 months of treatment; 6 months of follow-up.
Main outcome measure: Proportion of eyes with a ≥15-letter gain in BCVA.
Results: 60.2% of patients receiving aflibercept gained ≥15 letters compared with 22.1% of the sham group.
Reference: Holtz FG et al. Br J Ophthalmol. 2013;97(3):278-284. |
Choosing from a Plethora of Protocols
With such an embarrassment of therapeutic riches, how can clinicians best guide their patients? “This truly requires the ‘art of retina’ because there are so many variables to consider,” said Dr. Hariprasad.
Panning for gold. In the absence of head-to-head, well-designed trials with consistent criteria that clearly compare the current options, retina specialists have been left seeking a gold standard, said Dr. Fekrat. In the meantime, she said, treatment decisions are individually influenced by factors such as past experience with specific agents, availability, economics, and patient characteristics, including ability or willingness to travel for injections.
A lack of consensus remains, added Dr. Lin, about whether one therapy works better than another, when to switch from monthly to an as-needed (PRN) or treat-and-extend strategy, when to try different approaches or combination therapy, and how to manage the condition long term.
First-line monotherapy. Dr. Lin discusses all three anti-VEGF agents with her patients, explaining that Lucentis and Eylea are FDA approved for this indication and that Avastin is used off label.
Dr. Fekrat generally starts with Avastin. “If the patient’s macular edema has resorbed or resolved after two or three monthly injections, then I will follow a treat-and-extend approach to see how long the eye can go between treatment intervals. If the eye doesn’t respond after two or three injections, and the macular edema is still present on OCT, then I will consider switching to another anti-VEGF agent.”
Treating with Lucentis according to BRAVO and CRUISE, Dr. Campochiaro usually starts with six monthly injections, and then goes through a PRN phase to determine what the patient requires in terms of repeat injections.
Dr. Hariprasad offers patients all the FDA-approved options for RVO. “I tell them anti-VEGF therapy will give them excellent outcomes, but the treatment burden is much higher,” he said. “I also offer Ozurdex, explaining the risks and benefits compared with anti-VEGF therapy. Even though efficacy is lower, the treatment burden is less.”
Switching anti-VEGF agents. Because of the significant visual acuity benefits of anti-VEGF agents, Dr. Lin first makes a change within the anti-VEGF group before trying other types of treatment in patients who are not responding adequately. “My colleagues and I tend to choose Avastin or Lucentis first, then consider switching to Eylea for refractory cases or for patients who want to try to decrease the frequency of injections.” Some case reports are beginning to support the value of switching patients who have refractory CME on Avastin or Lucentis to Eylea, she said.
Combination regimens. “I’m a big believer in combination therapies,” said Dr. Hariprasad. “This disease is multifactorial, with both inflammatory and VEGF components. And although anti-VEGF therapies are the most powerful weapon we have against this disease, their durability is very poor, routinely requiring nine or ten injections in the first year.” Combining it with other therapies such as Ozurdex or laser can help decrease the treatment burden, he said.
Dr. Hariprasad starts some patients with Ozurdex monotherapy, checking them every six weeks, and touching up with Eylea or Lucentis as needed. “If the patient still has retinal swelling at the third or fourth month, I will inject another Ozurdex implant,” he said. “Along the way, I do laser treatment as indicated. With this regimen, I am likely to get a patient to two Ozurdex treatments and four or five anti-VEGF injections a year.”
Dr. Lin said that after failure of or incomplete response to at least two of the three anti-VEGF therapies in the first three months, she adds focal grid laser, an intravitreal steroid, or both.
Dr. McCannel combines Ozurdex (every three to six months) with anti-VEGF agents (every four to six weeks) in patients who don’t seem to respond well to either. “I get some pretty nice rescues out of that combination,” he said.
Dr. Fekrat follows similar drug combinations with grid pattern laser therapy, which offers a more permanent solution for the edema in BRVO than does medical treatment. “It’s easier to perform laser once the macular edema has resorbed because the laser is taken up better when the area is dry,” she said, noting that it may take a long time for the laser’s effect to “kick in.”
A critical period. Dr. Campochiaro considers two years the critical cutoff point for initiating additional treatments. “We’re finding that patients remain stable long term if they have a period of at least six months without requiring an [anti-VEGF] injection within two years of treatment initiation; but if they still require injections after two years, they are likely to continue to require them after five years. Therefore, if a patient is still requiring anti-VEGF injections two years after beginning treatment, I am willing to accept treatments that have more side effects as a trade-off for reducing treatment burden.”
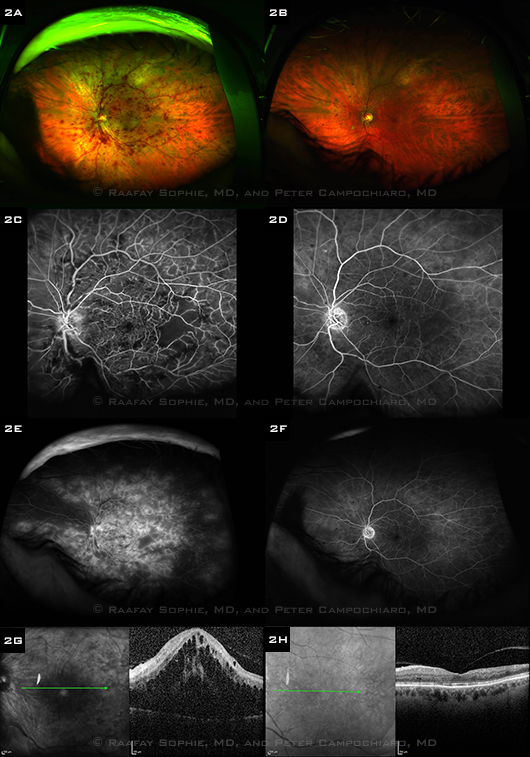 |
|
BEFORE AND AFTER TREATMENT. The left column shows images from a 77-year-old African-American man with a recent-onset CRVO, and the right column shows corresponding images one month after six monthly ranibizumab injections. (2A, B) Wide-angle fundus photos show resolution of the retinal hemorrhages and dilated vessels. (2C, D) Early-phase FAs show reperfusion of several areas of nonperfused retina. (2E, F) Late-phase wide-angle FAs show resolution of the severe, diffuse leakage. (2G, H) OCTs show resolution of subretinal and intraretinal fluid. Visual acuity improved from 20/200 at baseline to 20/25 after treatment.
|
Long-Term Management
Although it is possible to maintain visual acuity gains with PRN treatment, said Dr. Lin, there may be differences between BRVO and CRVO patients. Requiring follow-up every three months, the HORIZON trial revealed that visual acuity declined in CRVO patients who weren’t followed closely enough in the second year.7
“As a result, I’m a bit more vigilant with my CRVO patients,” said Dr. Lin. “If you get BRVO patients to a point of stability where you’ve managed to dry them out, you could conceivably have them come back every three months, whereas you may need to see your CRVO patients more frequently than that.”
Although early clinical trials pinpointed VEGF production as pivotal to the disease process, researchers and clinicians are just beginning to learn how to manage this condition over the long haul. “The next step,” said Dr. Campochiaro, “is to develop a means of achieving sustained suppression of VEGF in patients who still require injections two years after initiating treatment.”
__________________________
1 Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984;98(3):271-282.
2 Central Vein Occlusion Study Group M. Ophthalmology. 1995;102(10):1425-1433.
3 Haller JA et al. Ophthalmology. 2010;117(6):1134-1146.
4 Scott IU et al. Arch Ophthalmol. 2009;127(9):1115-1128.
5 Campochiaro PA et al. Ophthalmology. 2010;117(6):1102-1112.
6 Brown DM et al. Ophthalmology. 2010;117(6):1124-1133.
7 Heier JS et al. Ophthalmology. 2012;119(4):802-809.
Meet the Experts
PETER A. CAMPOCHIARO, MD Professor of ophthalmology and neuroscience, Wilmer Eye Institute at Johns Hopkins School of Medicine, Baltimore. Financial disclosure: Is a consultant (with remuneration to PAC) for Abbvie, Advanced Cell Therapeutics, Alimera, Applied Genetic Technologies, Gene Signal, and Kala and is a consultant (with remuneration to Johns Hopkins University) for Aerpio, Genentech, and Regeneron; receives research support for Johns Hopkins from Aerpio, Allergan, Genentech, Regeneron, Genzyme, and Oxford Biomedica; has equity ownership in Graybug.
SHARON FEKRAT, MD, FACS Associate professor of ophthalmology, Duke University School of Medicine; vitreoretinal surgeon, Private Diagnostic Clinic, Duke University Health System; Director, Duke Retinal Vein Occlusion Center of Excellence; Chief, Ophthalmology, Durham VA Medical Center. Financial disclosure: None.
SEENU M. HARIPRASAD, MD Associate professor of ophthalmology and visual sciences; chief of vitreoretinal service at University of Chicago Medicine. Financial disclosure: Is a consultant for Alcon, Allergan, Bayer, Leica, Ocular Therapeutix, OD-OS, Optos, and Takeda; and is on the speakers’ bureau for Alcon, Allergan, Genentech, and Regeneron.
PHOEBE LIN, MD, PHD Assistant professor of ophthalmology at Casey Eye Institute, Oregon Health & Science University, Portland. Financial disclosure: None.
COLIN A. MCCANNEL, MD Professor of clinical ophthalmology, Jules Stein Eye Institute, David Geffen School of Medicine, Los Angeles. Financial disclosure: None. |