By Roger P. Harrie, MD
Edited By: Ahmad A. Aref, MD, MBA
Download PDF
Note: This article has been updated since print publication. In the original article, EyeNet published the wrong images for Figs. 3A and 3B. EyeNet regrets that error. The correct images appear below.
Walter Williams,* aged 78, had been having trouble with his shoulder for some time, particularly at night, and he finally decided to undergo elective rotator cuff surgery.
A surprise before shoulder surgery. Mr. Williams underwent an electrocardiogram (EKG) prior to the surgery and was found to have atrial fibrillation. As he was asymptomatic, no treatment was started at that time.
Haze and “lightning” in the right eye. Four weeks later, Mr. Williams reported concerns about hazy vision in the right eye, which was associated with intermittent lightning streaks. His internist referred him for a magnetic resonance imaging (MRI) scan, which showed “chronic microvascular changes.” A carotid ultrasound and an echocardiogram were both reported as normal.
Next, trouble with the left eye. The vision in Mr. Williams’ right eye gradually improved, but 10 days later he experienced profound vision loss in his left eye.
What his ophthalmologist found. He was seen by his ophthalmologist, who documented vision of 20/60 in his right eye and light perception in his left.
At that time, the fundus examination revealed a small peripheral pigmented lesion in Mr. Williams’ right eye and changes in his left eye “consistent with ischemia.” The ophthalmologist presumed that Mr. Williams’ visual loss was due to a central retinal artery embolism, and the patient was referred to a cardiologist for anticoagulation therapy. The source of the embolus wasn’t established—given the normal carotid artery ultrasound and echocardiogram—so it was assumed to be due to his atrial fibrillation. The cardiologist started Mr. Williams on Eliquis (apixaban) for anticoagulation and suggested that he consider cardioversion for the arrhythmia. Because of the lesion in his right fundus, he was referred to our clinic for an ultrasound examination.
 |
|
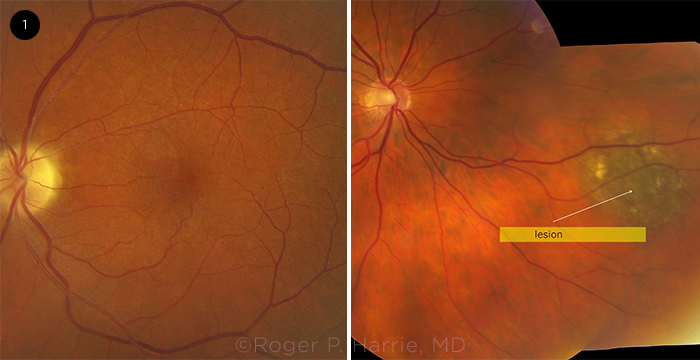
FUNDUS EXAM. In the patient’s right eye, we noticed a small peripheral, pigmented lesion (arrow). In his left eye, we noted optic disc pallor and vascular attenuation.
|
We Get a Look
Imaging and another eye exam. One week after the fundus exam, and four days after seeing the cardiologist, Mr. Williams came to our institute for ultrasound examination of the pigmented mass in his right eye. While in the office, he incidentally mentioned the recent visual loss in his left eye and expressed concern, as that had been his better eye.
Examination at that time revealed vision in his right eye of 20/60–2 with his glasses and bare light perception in his left. Examination of the pupils showed a pronounced left afferent pupillary defect. A slit-lamp examination was unremarkable except for bilateral pseudophakia. Intraocular pressure was 15 mm Hg in both eyes.
The fundus examination of his right eye revealed a small peripheral pigmented lesion, which was less than 1 mm thick by ultrasound. The examination of his left eye showed optic nerve pallor and attenuation of the retinal vessels (Fig. 1).
 |
|
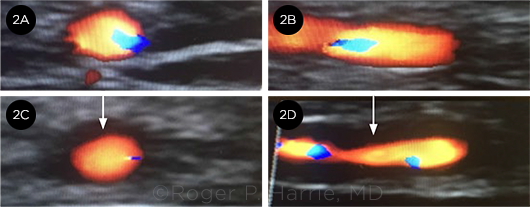
“HALO” SIGN. These images compare a (2A) cross-section and a (2B) longitudinal image of a normal temporal artery with the (2C) cross-section and (2D) longitudinal image of our patient’s temporal artery. Note the “halo” sign (arrows).
|
Making the Diagnosis
Mr. Williams stated that several doctors had told him that his vision loss was due to “a blood clot in the eye.” He was worried because the vision in his right eye continued to fluctuate and was bad enough on some days that he couldn’t drive—despite the fact that he was faithfully taking his prescribed blood thinner, in the hope that it would prevent the catastrophic vision loss that had already occurred in his left eye.
Checking his records. We reviewed his medical records, which confirmed the findings of a normal carotid ultrasound and echocardiogram as well as the diagnosis of atrial fibrillation.
His recorded medical history didn’t include any mention of headaches, scalp tenderness, or jaw claudication. When asked whether he had any such symptoms, he said that he had been experiencing severe headaches that required several ibuprofen tablets a day. He had problems chewing food to the point of changing his diet to soft foods and liquids, and he lost 10 pounds over two months. He told us that he didn’t have any scalp tenderness, but he did say that he had been having pain in his shoulder joints.
Imaging. The left eye was also examined by B-scan ultrasonography, which showed no embolic material in the central retinal artery. His temporal arteries were imaged with color doppler and demonstrated a bilateral “halo” sign, which was interpreted as inflammatory edema of the arterial walls (Fig. 2).
Lab work. His erythrocyte sedimentation rate (ESR) was obtained and the result was borderline at 36 mm/hour, but his C reactive protein (CRP) was reported as 81.4 mg/L (normal range is less than 3.0 mg/L).
Steroids prescribed. We referred Mr. Williams to his primary care doctor for the initiation of 1 gram of IV methylprednisolone for three days to be followed by high-dose oral prednisone.
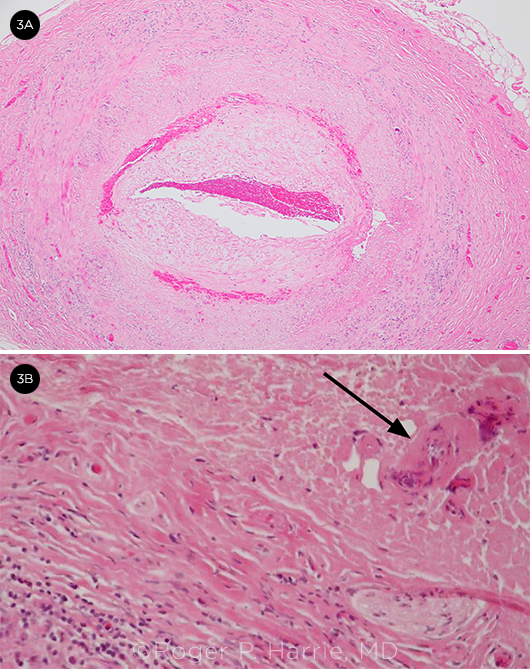
Biopsy. A temporal artery biopsy was performed three days later and was positive for GCA (Fig. 3). He had no improvement in his left eye, but the right eye stabilized at 20/60 without the fluctuations in visual acuity that he had experienced previously. His headaches and jaw pain have resolved.
 |
|
BIOPSY. (3A) Temporal artery biopsy with intima thickening and narrowing of the lumen and (3B) giant cells (arrow) and inflammatory cells.
|
Discussion
This case illustrates the importance of including GCA in the differential diagnosis of retinal artery occlusion.
Symptoms. Symptoms of GCA include headaches in 76% of patients, myalgia in 39%, visual symptoms in 37%, jaw claudication in 34%, and scalp tenderness in 31%.1
Testing. The ESR is an essential test to order when GCA is suspected,2 but it has a relatively low specificity. A rule of thumb for normal values for the ESR in men is to divide the age by 2 and for women to add 10 to the age and then divide by 2. In the case of Mr. Williams, his ESR was 36 mm/hour, which would have been within the normal range by this formula. However, by adding the CRP, which was highly elevated, the diagnosis was more certain. It is worth ordering both tests whenever you suspect a patient of having GCA.
Delayed diagnosis. Several doctors were misled by Mr. Williams’ diagnosis of atrial fibrillation. In elderly patients, the source of emboli in central retinal artery occlusion (CRAO) is most frequently associated with carotid artery plaques; in younger patients, with cardiac valvular lesions. A study by Yen et al. found that the adjusted hazard ratio was 8.32 for atrial fibrillation as the source of emboli and 5.34 for a carotid source, so patients should be evaluated with cardiac monitoring when other sources of emboli have been ruled out.3
A series of cognitive errors. In How Doctors Think,4 Jerome Groopman states, “… errors often occur not because doctors have inadequate knowledge or are incompetent,” but because “they have a tendency to get stuck in a particular mode of thinking.” He discusses three cognitive errors that may result in biased conclusions:
- Anchoring is defined as overvaluing the first data encountered, which skews thinking.
- Availability is defined as choosing the most likely diagnosis over less common ones.
- Attribution is defined as choosing the most plausible condition and not considering other possibilities.
Weighed down by anchoring, availability, and attribution errors. All of those cognitive errors occurred to some degree among the physicians who evaluated Mr. Williams. Only when a clinician outside the loop considered another possibility was the correct diagnosis made and appropriate therapy instituted.
___________________________
* Patient name is fictitious.
___________________________
1 Barraclough K et al. Br J Gen Pract. 2012;62(599):329-330.
2 Hale AJ et al. JAMA. 2019;321(14):1404-1405.
3 Yen JC et al. Biomed Res Int. 2015:374616.
4 Groopman JE. How Doctors Think. Houghton Mifflin Harcourt: 2007.
___________________________
Dr. Harrie is director of echography and an adjunct professor at the John A. Moran Eye Center at the University of Utah, Salt Lake City. Financial disclosures: None.