Download PDF
How often are patients misdiagnosed with glaucoma? “It happens more frequently than you might think,” said Steven D. Vold, MD, at Vold Vision in Fayetteville, Arkansas.
Kimberly Cockerham, MD, FACS, who practices in Stockton, California, agreed. “This is not something I see once in a blue moon. It is fairly common to see a patient who is on glaucoma drops and may not need them.”
Whether it’s glaucoma, an intracranial problem (such as pituitary adenoma, meningioma, or carotid or ophthalmic artery aneurysm), or an orbital problem (such as thyroid eye disease or an orbital tumor), certain cases can be a complex challenge for even the most experienced observer. But finding your way through the challenge is essential, as a misdiagnosis may lead to unnecessary testing and treatment. Even worse, it may seriously threaten the patient’s health or vision. Four experts offer guidance for sorting out the differences.
The History
Patient histories can offer clues to suggest there’s something other than glaucoma at play. Make sure these clues don’t go unnoticed, said Dr. Cockerham. Among her most baffling cases was a recent referral—a patient who was diagnosed as a “glaucoma suspect” decades ago and had been on eyedrops ever since.
Listen for clues. “He was a good historian,” she said, “but nobody had listened to him.” The patient described being hospitalized after a severe motor vehicle accident that resulted in a brain abscess. He recalled losing his visual field immediately after the accident and could provide specific details about which areas of his visual field were lost. He had had a completely stable visual field abnormality and optical coherence tomography (OCT) test results for years.
Consider age. Consider the patient’s age when taking the history and think about potential causes other than glaucoma, said Dr. Cockerham. “In a young patient, the cause is more likely hereditary, post-traumatic, inflammatory, or infectious. In middle age, compressive conditions and vascular events can occur. In older patients, giant cell arteritis can cause posterior ischemia that results in cupping and pallor.”
Nonglaucomatous problems that look like glaucoma can be asymptomatic. However, 1 common clue is sudden vision loss, which is typical of ischemic optic neuropathies, but not of glaucoma, said Dr. Vold. In contrast, compressive optic neuropathy tends to progress more gradually, confounding the diagnosis.
Watch symptoms, signs. Other symptoms and signs can help you begin to piece together the puzzle. The key is asking the right questions about vision, as well as asking probing questions about neurologic symptoms, said Prem S. Subramanian, MD, PhD, at the University of Colorado Health/Sue Anschutz-Rodgers Eye Center in Aurora, Colorado. “For example, loss of libido is a cardinal sign of some pituitary tumors in men, but patients often won’t volunteer this information.”
Ocular symptoms. Ask patients whether they have experienced any of the following symptoms:
- Sudden or quickly progressing vision loss
- Vision that’s different in only 1 eye
- Lack of color vision in 1 eye (red desaturation)
- Vision loss with eye movement
- Vision loss that came on with a severe headache
- Double vision
- Temporary graying or blacking out
- Orbital ache or pain
Neurologic symptoms. Ask whether patients have experienced any of the following neurologic symptoms or problems:
- Previous brain trauma or brain problem
- Numbness, weakness, or tingling
- Headaches, especially those that awaken them in the morning
- A loss of libido
Signs. Although optic disc pallor is a hallmark of a nonglaucomatous condition, said Dr. Subramanian, look for other signs like these as well:
- Proptosis, droopy eyelid, or facial asymmetry
- Loss of central visual acuity without a loss of peripheral vision
- Central scotoma or visual field that respects the vertical meridian
- Optic nerve pallor
- Optic nerves that are symmetric in appearance to each other, but 1 visual field is very different
- Unilateral or very asymmetric damage: afferent pupillary defect (APD)/color desaturation
- Conjunctival injection or chemosis
Ophthalmic Exam: Keep an Open Mind
Above all, be suspicious, said Dr. Cockerham. “Once a person gets a label of glaucoma, it often doesn’t get challenged, even when the patient ends up with a different doctor. The most suspicious diagnosis is unilateral normal-pressure glaucoma with an afferent pupillary defect. This is never the correct diagnosis.”
If a patient says they have glaucoma, make sure you agree, said Dr. Subramanian. “If something ‘smells funny’ or doesn’t quite fit, don’t be afraid to question another ophthalmologist’s diagnosis.”
A comprehensive ophthalmic exam. To confirm or rule out a diagnosis of glaucoma, Leah Levi, MD, at Scripps Health in San Diego, conducts a comprehensive ophthalmic exam.
“This includes checking acuity, color vision, pupils, and visual fields, and looking for eye movement problems,” she said.
“A patient with orbital problems may not be able to completely move his or her eyes in all directions,” added Dr. Cockerham. “Delegating the pupil and motility testing to your technician can be a problem.”
Testing intraocular pressure (IOP) is obviously important, said Dr. Vold, and if there are concerns about optic nerve head disease, additional visual fields may be needed. “A thorough vascular evaluation by an internist may be necessary to rule out uncontrolled diabetes or hypertension, and a fluorescein angiogram [may be needed] to spot a previous retinal injury from an old vein occlusion,” he said.
Look—with the light on. “The most confusing patient of all is one with a family history of glaucoma, no history of brain issues, and no symptoms whatsoever,” said Dr. Cockerham. If you suspect an abnormality, she said, turn on the light to see the patient’s eyes and face more clearly.
“A lot of eye specialists work in dim rooms, going from slit lamp to slit lamp,” she said. “We need to look at these patients in a fully lighted room to see if there is asymmetry of the face or globe position or evidence of bilateral involvement, like thyroid eye disease.” Other signs to watch for? “In a patient with a meningioma, for example, the temporal aspect of the face overlying the meningioma may get bigger,” she said, “and a carotid-cavernous sinus fistula will cause a characteristic dilation of the vessels on the surface of the eye, and eyelid swelling and proptosis.”
Palpate and measure. If you suspect an orbital problem, checking resistance to retropulsion can be helpful in detecting a mass or enlarged muscles behind the eye, said Dr. Cockerham. This is particularly helpful in Asian patients who do not become proptotic like other ethnicities. Dr. Levi also recommends measuring whether 1 eye is more proptotic than the other by using exophthalmometry, if available. Taking a photo from above can also be helpful, said Dr. Cockerham.
Visual fields. Any ischemic optic neuropathy can produce visual field defects similar to those seen in glaucoma, said Dr. Subramanian. Although certain patterns may raise glaucoma red flags, added Dr. Cockerham, visual field defects in a patient with a tumor and another with true glaucoma can be indistinguishable. “There’s nothing that’s pathognomonic.”
In addition, she said, digital perimetry is less clear than manual visual fields are in respecting the vertical meridian and in isolating a cecocentral scotoma. “There’s noise in the signal of automated visual fields,” said Dr. Cockerham. “The Humphrey visual field SITA testing, for example, fills in the information in between stimulus points, and this can mute neurologic visual field patterns that are more easily seen when a skilled technician has carefully plotted the Goldmann visual field.”
Still, automated visual fields can offer clues. For example, central loss is indicative of retina or optic nerve maladies, as opposed to glaucoma, said Dr. Vold. And in normal-tension glaucoma, patients usually don’t have visual acuity loss until later in the disease.
Whenever possible, it helps to look at visual fields of both eyes together, said Dr. Subramanian. “If you don’t look at them side by side, you may miss a homonymous visual field defect or even a bitemporal hemianopia. Your brain may fail to recognize the pattern if you don’t have both visual fields sitting in front of you at the same time.”
Fundus exam. “Over time, we’ve evolved to the point where people equate optic disc cupping to glaucoma,” said Dr. Cockerham. “But it is just 1 of many optic nerve processes that can cause cupping.” If the neuroretinal rim has pallor, it’s definitely a red flag that you are not simply dealing with glaucoma, said Dr. Levi. “With glaucoma, you may have cupping, but the actual surrounding rim is normal in color and looks healthy.” Spotting optic disc pallor is key to preventing a misdiagnosis, agreed Dr. Subramanian.
“In addition, with ischemic optic neuropathy, crowded or hypoplastic nerves are more common,” said Dr. Vold.
OCT. Because optic nerve fiber changes are not specific to glaucoma, OCT won’t be definitive in differentiating it from nonglaucomatous problems, said Dr. Levi, but an OCT scan may be helpful as a baseline for future follow-up.
“Because OCT is structural, however, it can provide a very clean delineation along a particular anatomic boundary,” said Dr. Subramanian. “That helps you to say, ‘I’m seeing damage here in a more diffuse pattern rather than the typical superior and inferior loss, and that makes me concerned this is something other than glaucoma.’”1
With glaucoma, said Dr. Vold, you’ll typically see inferior rim retinal nerve fiber layer loss before you see it anywhere else. “This area is usually affected first, then superior next, nasal third, and temporal last,” he said.
Even though certain patterns may be generally typical for glaucoma, they are not diagnostic, said Dr. Levi. For instance, if there is a tumor compressing the optic nerve from below, you will also get inferior RNFL thinning—so this finding is not specific to glaucoma and can’t be interpreted in isolation of the rest of the clinical picture. “Conversely, certain patterns are very atypical for glaucoma and should raise alarm bells.” These patterns include segmental RNFL thinning due to a loss of signal caused by media opacities, or sectoral peripapillary decrease in RNFL due to branch retinal vein occlusion.
In all patients but especially those under age 40, Dr. Subramanian also checks the source images for optic disc drusen, which can mimic glaucomatous defects.
Specialized imaging. A variety of red flags might warrant specialized imaging. Asymmetry may be one, said Dr. Cockerham, because glaucoma does not tend to be an asymmetric process. The following red flags indicate a nonglaucomatous problem is to blame, rather than glaucoma:
- The patient has unilateral normal-pressure glaucoma with an APD, especially if the APD is more than a subtle one.
- The patient has chronic open-angle glaucoma with an APD, especially if it’s more than subtle.
- The optic nerve is more pale than cupped.
- Visual field loss is progressing more rapidly than expected for glaucoma.
- Visual field loss is progressing despite normal IOP or IOP that’s under control.
- Severity of cupping doesn’t match the visual field defect.
- The OCT of the optic nerve and macula does not correlate with the visual fields.
- The visual fields or macular ganglion cell OCT have a vertical feel to them (homonymous pattern/ bitemporal/junctional).
- There are signs or symptoms of other nerve involvement, such as double vision or a droopy eyelid.
Signs and symptoms in synch? Another way to suss out nonglaucoma entities: “When making your assessment, don’t rely too heavily on any single particular piece of data and ignore others,” said Dr. Subramanian. Symptoms and signs need to align, emphasized Dr. Vold.
For example, it’s important to take note when a patient has an elevated IOP and some degree of vision loss—whether central visual acuity or a visual field abnormality—but the appearance of the optic disc doesn’t quite match, said Dr. Subramanian.
Or, in a patient with a potential pituitary tumoror other compressive lesion of the optic nerve or retrochiasmal visual pathway, comparing right and left eyes may reveal clues. “Analyzing the macular ganglion cell complex, you may see a pattern of ganglion cell loss that matches the visual field defect and can really demonstrate a homonymous or bitemporal defect,” he said. Many glaucoma specialists do not look at this testing and may miss that the problem is retrochiasmal, added Dr. Cockerham.
Refer to a Neuro-Ophthalmologist
If the clinical picture is not consistent with the degree of “glaucoma” you are seeing, it may be time to refer to a neuro-ophthalmologist, said Dr. Levi. What results in most referrals—and is most troubling for many general ophthalmologists and some glaucoma specialists—are patients who are losing visual fields despite what seems to be good control of their IOP. “Much of the time, patients referred to me do have glaucoma, however, and I can ascertain that by careful review of their clinical findings without getting a scan,” she said.
If needed, imaging may involve magnetic resonance imaging (MRI) or a magnetic resonance angiogram or computed tomography (CT) angiography. “If you have a high degree of suspicion and don’t feel comfortable reviewing these scans,” said Dr. Cockerham, “consider referring them to a neuro-ophthalmologist.”
Dr. Cockerham cited the case of a patient where this didn’t happen. The patient had been seen by 5 previous eye care providers, but over the course of 6 months she lost vision in the involved eye to no light perception. In this patient, the noncontrast CT scan of the brain was done in an emergency department and had been read as normal, but an apical mass was visible on 1 digital slice. An MRI with gadolinium revealed a large orbital apex mass that was found to be steroid-responsive, but there was no return of vision.
___________________________
1 Gupta PK et al. Open Neurol J. 2011;5:1-7.
Glaucoma Plus
A Case of “Ticks and Fleas on the Same Dog”
A 70-year-old woman was referred to Dr. Levi’s clinic with chronic visual loss. Her medical history included hypertension, obstructive sleep apnea, well-controlled diabetes, and breast cancer that was treated in 1999 and was in remission.
Her ocular history included laser and cryotherapy in each eye in the 1990s for retinal holes due to lattice degeneration. She had cataract extraction in her right eye in 2005 and in her left eye in 2011.
The patient began to notice a cloud in the vision of her left eye in 2010. This progressed over several months. She was told by a glaucoma specialist that she had normal-tension glaucoma that was worse in the left eye than the right, and he started her on latanoprost.
She was then lost to ophthalmological follow-up but her primary care physician apparently continued to refill the drops. Over the next 2-3 years, she gradually lost vision in the left eye. In 2014 she began to notice visual changes in the right eye and returned to the retina specialist who had seen her in the 1990s. In July 2014, her IOP was 24 mm Hg in each eye. She was placed on brinzolamide/brimonidine drops and was referred for neuro-ophthalmological evaluation.
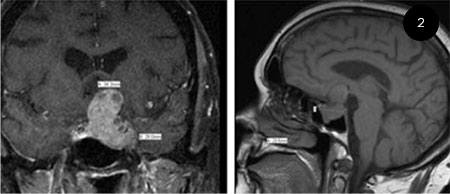 |
On initial neuro-ophthalmological evaluation in August 2014, acuity was 20/30 in the right eye and bare light perception in the left. Hardy Rand and Rittler (HRR) color plates was 3/6 in the right eye. Visual field testing showed a dense superior arcuate defect in the right eye and the mean deviation was –11.61, and no responses in the left (Fig. 1). There was a left relative afferent pupillary defect. There was no clinical evidence of Horner syndrome. Extraocular movements were full. Trigeminal nerve function, including corneal sensation, was symmetric and normal. IOP was 14 mm Hg in each eye. There was 0.8 cupping of the right disc with pallor of the remaining neuroretinal rim. The left disc was completely cupped. Because of the pallor of the neuroretinal rim in both eyes, an MRI scan was done; it showed a large sellar mass with suprasellar extension and left cavernous sinus invasion (Fig. 2).
 |
In September 2014 the patient underwent subtotal resection of the mass, which proved to be a pituitary adenoma. The resulting decompression of the anterior visual pathways led to improvement of the color vision to 4.5/6 in the right eye. Visual field testing in the right eye improved; the mean deviation in the right eye improved to –4.43. In addition, a superior vertical step was revealed reflecting the chiasmal compression (Fig. 3). The left eye did not improve. The patient’s last examination in June 2018 was stable as was her MRI scan. She has continued to use the drops in her right eye.
Take-Home Lessons
- Patients with glaucoma need to be followed by an ophthalmologist. This patient with glaucoma was lost to ophthalmological follow-up for about 3 years while she progressively lost vision, but her primary care physician continued to prescribe her glaucoma drops.
- More visual field loss than expected. This patient had more visual field loss than expected for the degree of cupping as well as faster progression of visual loss than expected for glaucoma, suggesting a nonglaucomatous condition.
- Pay attention to pallor. Uncommonly, compression of the anterior visual pathways can produce cupping that is similar to glaucoma, but in these patients the remaining neuroretinal rim will show pallor. The pallor in this case indicated that the patient had a chronic nonglaucomatous optic neuropathy in addition to glaucoma. An MRI scan was therefore indicated.
Meet the Experts
Kimberly Cockerham, MD, FACS Orbit-plastics-neuro-ophthalmology specialist in private practice in Lodi, Modesto, and Stockton, Calif.; Adjunct Associate Clinical Professor at Stanford University, Palo Alto, Calif. Relevant financial disclosures: None.
Leah Levi, MD Clinical Professor Emerita at the University of California, San Diego; and director of neuro-ophthalmology at the Scripps Clinic Division of Ophthalmology in San Diego. Relevant financial disclosures: None.
Prem S. Subramanian, MD, PhD Professor of ophthalmology, neurology, and neurosurgery and division head of neuro-ophthalmology at the University of Colorado Health/Sue Anschutz-Rodgers Eye Center, Aurora, Colo. Relevant financial disclosures: None.
Steven D. Vold, MD Glaucoma and cataract specialist at Vold Vision in Fayetteville, Ark. Relevant financial disclosures: None.
Full Financial Disclosures
Kimberly Cockerham, MD, FACS None.
Leah Levi, MD None.
Prem S. Subramanian, MD, PhD GenSight Biologics: S; Quark Pharmaceuticals: L,S; Santhera Pharmaceuticals: S.
Steven D. Vold, MD Aerie Pharmaceuticals: C,S; Alcon: C,S; Allergan: L,S; Alphaeon: C,O; Bausch + Lomb: S; Carl Zeiss Meditec: C,S; Diopsy: C; Glaukos: C,S; InnFocus: C,S; Iridex: C,S; iSTAR Medical: C; Ivantis: C,S; Lumenis: C; NeoMedix: L; Ocular Therapeutix: C,S; RxSight: S; Transcend Medical: C,P,S; TrueVision Systems: C; Volk Optical: P.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|