Download PDF
The mucosal surface of a healthy eye is awash in a stew of antimicrobial molecules and immune cells. But, under homeostatic conditions, the bacterial species Corynebacterium mastitidis (C. mast) can thrive there harmlessly while also boosting the eye’s immunological defenses against other infections, researchers have discovered.
In a series of mouse experiments, a team of scientists at the National Eye Institute (NEI) found that C. mast induced T-cells in the ocular mucosa to produce interleukin-17 (IL-17), which controls the local production of antimicrobial molecules. In turn, this prevented invasive surface infections of Candida albicans and Pseudomonas aeruginosa.1
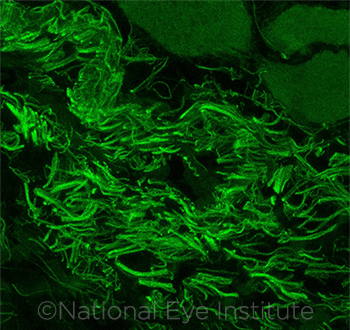 |
IMMUNE RESPONSE. C. mast, found on the murine ocular surface and fluorescently labeled in this image, stimulates a beneficial immune response in the ocular mucosa—and is not eradicated from the eye.
|
Ocular microbiome. There has been increasing recognition in recent years that commensal bacteria—the “microbiome”—play important roles in localized immune processes of the skin, gut, and other organ systems, said coauthor Rachel R. Caspi, PhD. But there was some doubt that commensal bacteria could survive on the ocular surface, she said.
“There was really no consensus that anything could live there, because the surface of the eye is highly antibacterial. We have lysozyme in tears. We have antibacterial peptides and other substances. And neutrophils come out onto the surface of the eye and patrol the eye for pathogens,” she said. “What we were able to find is that the bacterium that we have identified actually lives on the ocular surface for the long term.”
Persistence on surface. In mice, the NEI researchers found, C. mast is transmitted from mother to pup, but it is not passed between adult animals. This supports the notion that C. mast actively colonizes the tissue and is not continually reinoculated from the skin, said Anthony St. Leger, PhD, lead author of the study.
“The C. mast colonizes the eye very early in life, before the immune system has a chance to mature. Despite the bacterium stimulating an immune response, somehow it has evolved a way to avoid being eradicated from the eye,” he said.
Next steps. In addition to determining how the bacteria persist, the researchers plan to investigate the impacts of antibiotic medications on the commensal system. “We treated the mice with antibiotics and it effectively killed the bug, but it suppressed that immune response as well. So I think our study highlights the need to better understand how topical and systemic antibiotics manipulate the ocular microbiome,” Dr. St. Leger said.
“For the moment, we’ve just stopped at the point of finding that taking this bacterium away leaves the eye open to pathogenic infections,” Dr. Caspi said. “We have not gone beyond that to try to see if we leave these mice alone now if this bacterium is going to come back, and maybe even become antibiotic-resistant.”
From mice to people. It is too early to know if C. mast or another microbe might also function as a commensal in the human eye. However, it is likely, as Corynebacterium species are routinely found in conjunctival swabs of humans, said coauthor H. Nida Sen, MD, MHSc.
“I think the relevance to the human ocular surface has to be established first,” Dr. Sen said. “But conceptually, as a [research] group, we are interested in what this might mean for human inflammatory diseases or ocular surface diseases in general. Manipulation of the microbiome has the potential to shift the paradigm in treatment and prevention of disease.”
For instance, perhaps contact lens–related infections could eventually be treated with commensal eyedrops that boost the localized immune system, Dr. Caspi said. “We probably don’t want to be instilling live bacteria into the eye. So we would want to test preparations—either an attenuatation or, more simply, an extract from the bacterium —for their ability to replace the whole entire bug. The goal would be to mimic a commensal bacterium, but it would be under our control,” she said.
—Linda Roach
___________________________
1 St Leger AJ et al. Immunity. 2017;47(1):148-158.e5.
___________________________
Relevant financial disclosures—Drs. Caspi, Sen, and St. Leger: None.
For full disclosures and disclosure key, see below.
More from this month’s News in Review