Download PDF
This month, News in Review Highlights selected papers from the original papers sessions at AAO 2017. Each was chosen by the session chair because it presents important news or illustrates a trend in the field. Only 4 subspecialties are included here; papers sessions will also be held in 6 other fields. See the Meeting Program, which you’ll find in your meeting bag, or the Mobile Meeting Guide for more information.
A method for using femtosecond laser to change the refractive power of an already implanted intraocular lens (IOL) appears to be biocompatible, with no evidence of postoperative inflammation or toxicity, a preclinical study has found.
In the technique, a specialized, low-power femtosecond laser (Perfector, Perfect Lens) alters the lens power by making specific parts of a hydrophobic acrylic lens more hydrophilic, said Nick Mamalis, MD, at the University of Utah in Salt Lake City.
“The reason why this technology is so interesting is that it allows the change of power of an IOL that is already inside the eye. This doesn’t require a special lens to be placed to begin with,” said Dr. Mamalis.
Precise femtosecond laser nomograms for different types of corrections—including spherical power, astigmatism, and multifocal adjustments—were developed previously ex vivo, Dr. Mamalis said. This study was designed to test the safety and effectiveness of such treatments in pseudophakic, living eyes, Dr. Mamalis said.
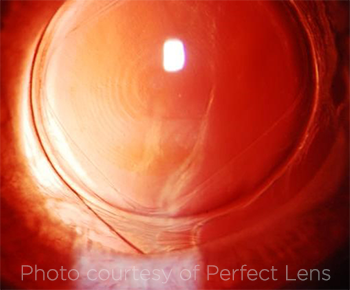 |
TREATED LENS. This slit-lamp image is of an IOL in a rabbit eye. What looks like a multifocal pattern—visible individual zones in the IOL—is a refractive change. This is done with a phase wrapping algorithm and allows higher diopter changes.
|
Study details. In the study, 6 rabbits had a standard hydrophobic acrylic IOL implanted in both eyes (with the fellow eyes as controls), followed by a 2-week healing period. Then the laser treatments were performed in 1 eye.
“We followed the rabbits for an additional 4 weeks, to look for signs of inflammation or toxicity from the laser treatment,” Dr. Mamalis said. “We then sacrificed the animals and examined the enucleated eyes grossly and histopathologically.”
No inflammation. In slit-lamp examinations, the researchers found “no postoperative inflammation or toxicity in the treated eyes,” he said. “More importantly, when we looked at them in terms of a careful histopathologic examination, there was no sign of untoward inflammation, toxicity, or any kind of a reaction in the eyes that had the laser adjustment done, compared to the control eyes.”
Adjustments on target. The researchers then explanted the IOLs in order to assess the degree to which they had been adjusted. “What we found is that the treatments were incredibly precise and that the changes in power were really consistent, within 0.1 D of target,” Dr. Mamalis said.
| Evaluation of the Biocompatibility of IOL Power Adjustment Using a Femtosecond Laser. When: Tuesday, Nov. 14, 10:00-10:07 a.m., during the second cataract original papers session (10:00 a.m.-noon). Where: Room 271. Access: Free. |
Clinical implications. If this technology eventually is proved safe and effective in humans, it would help cataract surgeons optimize patients’ postoperative visual acuity, Dr. Mamalis said. “Oftentimes we calculate everything correctly and still have a refractive surprise, especially in patients who have had previous refractive surgeries. This would allow us to correct those [cases],” he said.
—Linda Roach
___________________________
Relevant financial disclosures—Dr. Mamalis: Alcon: S; Anew Optics: C,S; Calhoun Vision: S; ClarVista Medical: S; Cord: S; LensGen: S; Medicontur: S; Omega: S; Perfect Lens: S; PowerVision: S; Shifamed: S; Zeiss: S.
For full disclosures and disclosure key, see below.
More from this month’s News in Review