Download PDF
No ophthalmologist has experience with all corneal dystrophies—let alone all the genetic disorders of the cornea, said Jayne S. Weiss, MD, at Louisiana State University in New Orleans. Consequently, these disorders may be challenging to diagnose at first.
“Before you operate on a patient with a corneal dystrophy, you really need to understand it,” said Dr. Weiss. It’s important to appreciate the depth and location of the disease and to realize that surgery may exacerbate the condition in an atypical way, she said.
What steps should a surgeon take? If you suspect a hereditary condition, said Dr. Weiss, you might take photos of the patient’s cornea and send the pictures to a specialist who has more familiarity with these types of diseases.
Another approach is to turn to the International Classification of Corneal Dystrophies–Edition 2 (IC3D2),1 available for free at the Cornea Society website (www.corneasociety.org/publications/ic3d), she said. There, you can find approximately a page of information about each type of dystrophy with clinical photos and findings as well as background on genetics.
To provide a sense of some of the challenges involved, five cornea experts discuss examples of corneal dystrophies and other genetic corneal disorders that can lead to surgical surprises—and what you can do to avoid operating in the dark.
 |
|
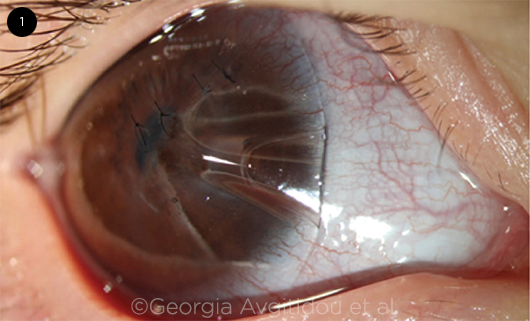
BRITTLE CORNEA SYNDROME. Spontaneous corneal perforation in a child with BCS. To repair it, four corneal sutures were placed and the eye was covered with a contact lens.
|
Brittle Cornea Syndrome: A Big Risk for Rupture
Brittle cornea syndrome (BCS) is an autosomal recessive connective tissue disorder that causes severe corneal thinning and carries an increased risk for spontaneous perforation or rupture from minimal trauma.2
BCS is a more accurate name for a group of conditions that previously were labeled keratoglobus and that are sometimes mistakenly lumped in with keratoconus—both also characterized by corneas that are abnormally thin, said Deborah S. Jacobs, MD, at Harvard Medical School in Boston. “As we got a better understanding of the molecular abnormalities involved and the genetic commonalities,” said Dr. Jacobs, “it became clear that many of these syndromal disorders were linked—and are better classified as brittle cornea syndrome.”
Diagnosing BCS. Presenting early in life, BCS tends to create a pattern of bilateral thinning from limbus to limbus, said Dr. Jacobs. She added that tomography, rather than topography, can be helpful with diagnosis. In addition, the sclera appears blue because it is so thin that you can see the choroid through it, said Ken K. Nischal, MD, FRCOphth, at the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh.
“If you see a thin cornea that looks like it is protruding, stop and ask yourself, ‘Could this be BCS?’” said Dr. Nischal. Because both parents could carry one mutated gene without manifesting abnormalities clinically, you have to do genetic testing to confirm the diagnosis, he said.
Risks of BCS. With brittle corneas as thin as 200 μm, children with BCS can easily experience a rupture of the eye with only minimal trauma, said Dr. Nischal. He described children under his care who had experienced ruptures—one whose eye was hit by a paper ball and another with a pen, and another who accidentally poked herself in the eye.
Surgical precautions. With such fragility, the eye behaves atypically on the operating table, said Dr. Jacobs. “The tissue is floppier, so it’s like suturing butter. Because it’s hard to seal wounds, the eye leaks post-op and is often prone to infection and rupture.”
Dr. Nischal offers two surgical precautions: “First, when making an incision in the eye, make it smaller than you want because the incision will expand,” he said. “For example, if you want a 20-gauge entry, use a 25-gauge needle.”
Second, reduce pressure in the eye. Because the cornea is thin and there is no rigidity at the limbus, pressure will always read low, even though it is actually high, he said. “I invariably start these children on glaucoma medication to make sure they don’t get a spontaneous rupture because of high pressure. Also, by reducing the pressure in the eye, you allow the cornea to be a little thicker.”
What about CXL? Corneal cross-linking (CXL) is used to prevent further thinning in keratoconus, so the question has arisen: Should it ever be used in children with BCS? Dr. Nischal would never consider it. There is a report of a single case that was successful,3 said Dr. Jacobs, adding that penetrating surgery tends to not go well. “Surgeons need to get better at identifying who to cross-link, who to operate on, and who to offer more creative techniques,” she said. (For a cautionary case report about atypical keratoconus, see below.)
Corneal grafts. An option for everyday protection of the eyes is to place an overlay graft, said Dr. Nischal. This involves removing the epithelium from the host and placing a donor Descemet and endothelium overlay graft from limbus to limbus.
“Beforehand, you should perform a paracentesis to lower the pressure in the eye,” he said. “That way, the tissues will appose each other when you suture on the overlay.” In many cases, added Dr. Nischal, it’s necessary to insert a tube shunt to manage eye pressure.
Sometimes an overlay graft may allow you to later do a full-thickness corneal transplant to improve vision, added Dr. Jacobs.
 |
|
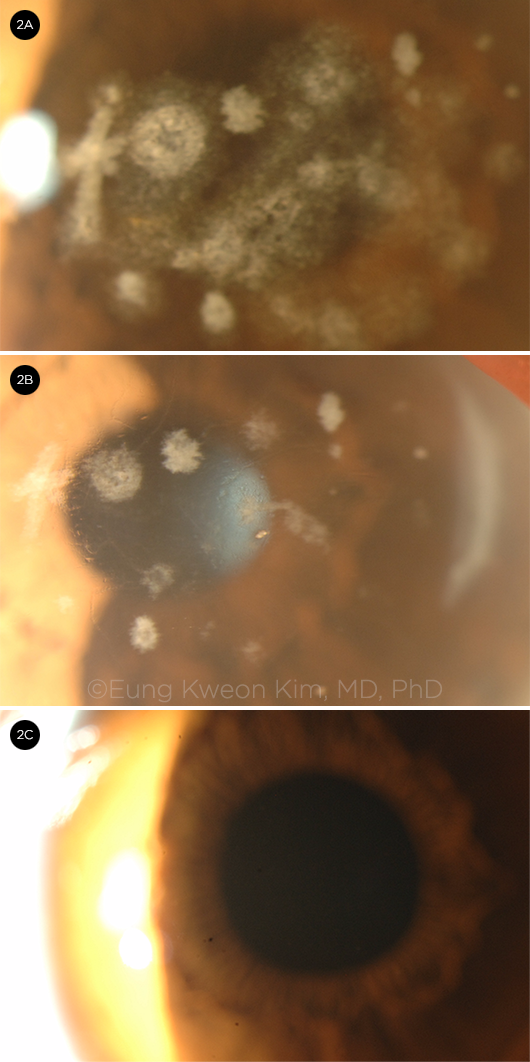
GCD2. A 67-year-old woman with (2A) granular deposits, linear lesion (latticelike lesion), and diffuse haze; (2B) after some granular deposits were removed. (2C) A 23-year-old genetically heterozygote woman with no deposits.
|
Granular Corneal Dystrophy, Type 2: An Evolving Presentation
Granular corneal dystrophy, type 2 (GCD2) is a rare autosomal dominant genetic disorder caused by a mutation in the TGFBI gene. Although TGFBI dystrophies affect multiple layers of the cornea, irregularly shaped but well-demarcated granular deposits appear mainly in the superficial central corneal stroma in GCD2, leading to progressive visual impairment.
Presentation in children. Early cases of GCD2 may show up as an unimpressive subepithelial opacity, said Dr. Weiss, and look very different from full-blown disease. This is why having an understanding of early disease is important.
“In children, a TGFBI-related dystrophy will often present like dry eye or Thygeson superficial punctate keratitis,” said Dr. Nischal. “If you have made that diagnosis in a child and treatment is not working as expected—but you see discrete spots within a localized faint hazy cornea—have a low threshold of suspicion for GCD2.” However, added Eung Kweon Kim, MD, PhD, it’s important to realize that these signs may not show up in a heterozygote who is age 8 or younger.4,5
Diagnosis. A slit-lamp exam and/or gene test can help diagnose the condition, said Dr. Kim, at Yonsei University in Seoul, South Korea. “First look at the cornea through the slit lamp,” he said, but recognize that GCD2 shares a similar morphology with other genetic conditions. “That’s why genetic testing is sometimes needed to confirm the diagnosis and is especially recommended before refractive surgery when deposits are not visible.”
Dr. Kim said that a test called AvaGen (Avellino; about $300) examines more than 70 mutations of the TGFBI gene for corneal dystrophies in addition to testing 1,000+ variants for keratoconus.6
Dr. Kim also uses an OCT scan of the cornea to measure the thickness of deposits before performing phototherapeutic keratectomy or lamellar keratoplasty.
Surgical contraindications. “Do not perform surgery through the central cornea,” said Dr. Kim. “This can exacerbate the deposits in GCD2 after procedures such as photorefractive keratectomy, LASIK, and LASEK.”
“Cutting a flap during a LASIK procedure, for example, will cause the GCD2 to progress and opacities to worsen,” said Dr. Weiss. Many deposits occur along the interface between the posterior surface of the flap and the surface of the remaining posterior stroma, said Dr. Kim. “If you discover GCD2 during a LASIK procedure, do not go forward with LASIK on the second cornea.”
Other types of surgery. If LASIK has been performed, phototherapeutic keratectomy (PTK) may improve corneal transparency and visual acuity.7 “It is better to amputate a LASIK flap than to preserve it,” said Dr. Kim.
It is also sometimes possible to postpone a keratoplasty by performing PTK on these patients, he said. PTK may be used judiciously to reduce the visually significant stromal haze, added Dr. Weiss. “However, with repeated PTK, eventually kerotoplasty may be required. Therefore, it’s important to understand what the laser can and cannot do, and not to promise the patient too much.” For example, after PTK, there can be delayed healing at the superficial cornea, where the previously deeply located deposits remain and are finally exposed to the ablated surface, said Dr. Kim. And you are more likely to end up with more scarring after PTK than you would with a different type of mutation, added Dr. Nischal.
DALK. Because the endothelial layer is always normal in GCD2, a relatively deep anterior lamellar keratoplasty (DALK) is better than a penetrating keratoplasty, said Dr. Kim. Also consider DALK instead of PTK for this particular mutation, said Dr. Nischal. “To prevent scarring after a corneal transplant, consider removing sutures sooner than you normally would.”
 |
|
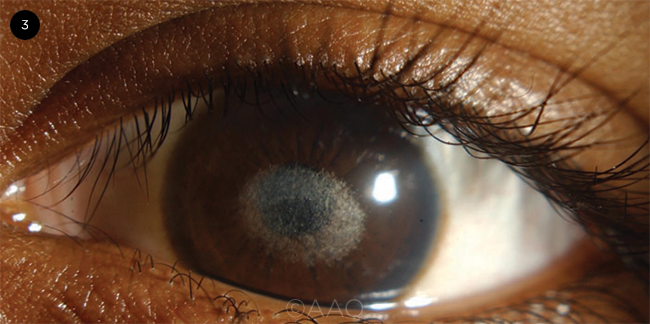
SCHNYDER CORNEAL DYSTROPHY. Corneal crystals in a girl with Schnyder corneal dystrophy.
|
Schnyder Corneal Dystrophy: Crystals May Not Be Present
Schnyder corneal dystrophy is an autosomal dominant eye disease leading to abnormal deposits of cholesterol and phospholipids in the cornea (Fig. 3). It has always been classified as a stromal dystrophy. However, Dr. Weiss has surgically removed the cornea and found lipid deposits not only throughout the stroma, but also in the base of the epithelium and in some endothelial cells.
Although the disease used to be called Schnyder crystalline corneal dystrophy (SCCD), only about half the people with the condition have crystals, which are superficial underneath the epithelium, said Dr. Weiss. “In the past, those without crystals never got diagnosed,” she said, explaining that this prompted creation of the IC3D to initiate a name change for the disease.
“The disease is poorly understood by most ophthalmologists,” said Dr. Weiss, “but it’s critical to know how and where it occurs to manage it surgically the right way.”
Signs of Schnyder. All Schnyder patients develop a front-to-back opacification of the cornea with time, said Dr. Weiss. Characteristic findings based on age are as follows:
- Age 23 or younger: All have a central opacity and/or central subepithelial crystals.
- Between about ages 23 and 38: Patients also develop the arcus lipoides at the corneal periphery.
- Older than about 38: A formerly clear area between the central opacity or crystals and the peripheral arcus becomes cloudy—more so at the center and periphery. Patients may also have decreased corneal sensation, said Dr. Weiss.
Diagnosis. If you simply examine the patient straight on, sometimes the whole cornea looks hazy, said Dr. Weiss. However, it can help to dilate the pupil and use retroillumination at the edge of the cornea. This will highlight opacification at the center and periphery, revealing a less-opacified, donut-shaped area in between.
“If you know what you are looking for, Schnyder is as obvious as the day is long,” said Dr. Weiss. “If you don’t, genetic testing may be a good idea. Also, ask if anyone in the family has had cloudy corneas. This can help you make the diagnosis.”
Cholesterol testing. Although it’s not diagnostic, cholesterol testing is a good precaution to take before surgery, said Dr. Nischal. “In adults, I would advise both a lipid screen and an examination to confirm that arteries are healthy.”
Dr. Weiss suggests that both affected and unaffected members of Schnyder pedigrees obtain serum lipid testing because of increased prevalence of hyperlipidemia in these families.
Surgery. Addressing disease surgically includes:
- Removing crystals. “If crystals are in the visual axis, often the ophthalmologist’s focus is to remove them, which can be done with PTK,” said Dr. Weiss. “But if you are not familiar with the other feature of Schnyder—the opacification of the cornea from front to back—you may be surprised, and the patient may be disappointed to find that vision remains poor even after crystals have been removed.”
- Addressing opacification. When central opacification is significant, said Dr. Weiss, it may prompt a discussion: Do you do a DALK, taking out the majority of the cornea and leaving the endothelium? Or do you do a full-thickness corneal transplant? Helping to inform this decision is the knowledge that any dystrophy can recur over time, moving from the periphery to the center, she said.
 |
|
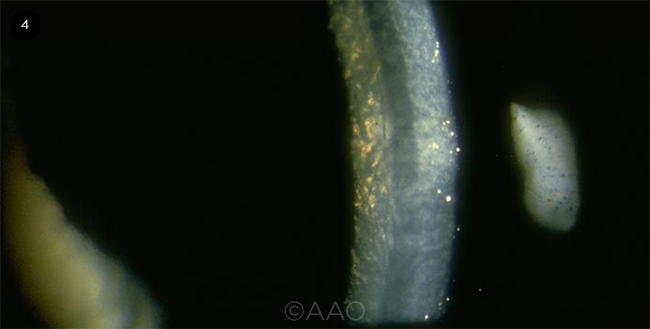
FUCHS DYSTROPHY. Corneal guttae in a Fuchs patient.
|
Fuchs Dystrophy: Preventing Progression, Ensuring a Better Outcome
In contrast to other dystrophies, Fuchs is an abnormality of the corneal endothelium, leading to corneal decompensation, said Dr. Weiss. “It’s important to distinguish between patients who just have corneal guttae and those who have a family history or who progress from corneal guttae to corneal edema, which is Fuchs.”
Surgeons can successfully treat Fuchs with replacement of the corneal endothelium—the most common reason for this type of surgery in the United States, said Kathryn Colby, MD, PhD, at the University of Chicago. However, there are key steps to ensure better outcomes for Fuchs patients undergoing either cataract surgery or corneal replacement.
Cataract surgery. Fuchs is more common and more easily identified than other types of genetic diseases of the cornea, said Dr. Jacobs. “However, if you don’t recognize it before you perform cataract surgery, you may speed progression of the disease.”
Cataract surgery is more challenging in patients with Fuchs dystrophy, said Dr. Colby. “The corneal guttae impair the view into the eye, the Descemet membrane is prone to detaching during surgery, and corneal edema is common after surgery, slowing visual recovery.” During cataract surgery, be careful with the endothelium and Descemet membrane, consider near-clear incision to reduce endothelial cell loss, and be parsimonious with phaco power, said Dr. Colby.
Preop considerations. Advice for better outcomes after cataract surgery includes:
- Tomography. A recent paper in Ophthalmology8 shows that tomography can identify several features that may put patients at greater risk of progression after cataract surgery, said Dr. Colby. These features include loss of regular isopachs, nasal displacement of the thinnest point, and posterior surface depression. The researchers found that when two or three tomographic features of subclinical edema are present—versus none or only one—there was a several fold–increased risk of disease progression over a median of five years. “Before this paper came out, I relied on the thickness of the cornea, which wasn’t the best measure due to baseline variability in the general population,” Dr. Colby said. Now, however, she relies on tomographic features to assess prognosis in Fuchs patients. “If they have no features, they are unlikely to need a corneal transplant after cataract surgery. If they have one feature, they are at intermediate risk of needing a transplant. If they have two or three features, they are at higher risk.”
In addition to tomography, specular microscopy and endothelial cell count may be helpful in preparing physicians and counseling patients, added Dr. Jacobs.
- IOL selection. Because the eye must have perfect optics to produce good results, Dr. Colby suggests exercising extreme caution about putting a multifocal lens in a Fuchs patient. She’s seen cases in which the cataract surgeon didn’t notice that the patient had Fuchs and put in an expensive multifocal. Besides being unhappy about the cost and outcome, she said, patients sometimes ask, “Did the cataract surgery cause my Fuchs?”
Corneal replacement. There are a few options.
- Corneal transplantation. “All evidence suggests that Descemet membrane endothelial keratoplasty (DMEK) provides the quickest visual recovery,” said Dr. Colby. “People achieve almost the same level of vision with a Descemet stripping endothelial keratoplasty (DSEK), but visual recovery is quicker with DMEK.” However, in 2018, nearly two-thirds of all EKs were DSEK, she said, likely because DMEK is more technically demanding. (See “Evaluation and Management of Fuchs Dystrophy,” for more Fuchs surgeries.)
- Descemet stripping only (DSO). Six years ago, Dr. Colby performed her first deliberate Descemet stripping only (DSO) procedure. This involves removing the Descemet membrane and endothelium and allowing the peripheral endothelium to grow back and rejuvenate the cornea.
Rho kinase (ROCK) inhibitors have been used as an adjunct therapy for DSO. Preliminary evidence suggests that they help increase the final endothelial cell count and speed the healing process, said Dr. Colby. Although ripasudil eyedrops are not FDA approved for use in endothelial rejuvenation, patients can order them online for use postoperatively. It’s a ROCK inhibitor approved in Japan as a glaucoma treatment, she said. There has also been some off-label usage of topical netarsudil, currently approved in the United States for treatment of glaucoma, to promote endothelial rejuvenation, she said.
However, Dr. Colby cautions ophthalmologists that online patient communities are recommending use of ROCK inhibitors without removal or destruction of the endothelium. “From a scientific standpoint, this makes no sense,” she said. “The cells of the endothelium are contact inhibited. Once they touch each other, they stop moving around. Unless you physically get rid of some of the cells—either by freezing or removal—the healthier peripheral cells will not be able to migrate because there is nowhere for them to go.”
Atypical Keratoconus: A Case Report
Dr. Jacobs was involved in the care of a patient who was initially thought to have keratoconus.1 The patient had diffuse corneal thinning and progressive steepening, but she had experienced a change in vision in her 50s—a little late for this kind of progression, said Dr. Jacobs. The patient was offered CXL.
Spontaneous ruptures. “Although [post-op management of] the case was uncomplicated at first, a spontaneous perforation occurred two weeks after surgery,” she said. “After a surgeon repaired the perforation, I became involved in the care of the patient for visual rehabilitation.”
When the other eye began to worsen, the patient sought the opinion of many specialists, and she ultimately chose CXL again. “The second procedure was done using epi-on,” said Dr. Jacobs. This “may be less effective but is thought to be less invasive, reducing the risk of perforation. However, the second eye also perforated in the second week post-op.”
Atypical presentation. Although this patient first appeared to have keratoconus, said Dr. Jacobs, the unusual presentation and morphology were closer to keratoglobus—without keratoconus’ usual gradient of thinning at the apex and normal thickness at the limbus—which led her team to investigate the family’s genetics.
“In addition to corneal thinning, the patient and her daughter both had three different genetic variants on a gene that is implicated in brittle cornea syndrome,” said Dr. Jacobs. “Although this case is not strictly considered BCS, which is autosomal recessive, the BCS gene is involved, and it seems to be playing a role in corneal thickness and stability after trauma or surgery.”
A cautionary tale. If you see someone with an atypical keratoconus—rapid progression in the first decade but thinning that’s more suggestive of keratoglobus—be hesitant about cross-linking, said Dr. Jacobs. “We can sometimes safely cross-link under 400 μm by using riboflavin with a different osmolarity,” she said, “but if it’s below 400 μm and equally thin at the limbus, then think hard about what the patient might have. Look for syndromal features, such as blue sclerae, deafness, or skeletal abnormalities, and consider a genetic evaluation.”
___________________________
1 Zhang W et al. Cornea. 2019;38(8):1033-1039.
|
___________________________
1 Weiss JS et al. Cornea. 2015;34(2):117-159.
2 Wright E et al. Orphanet J Rare Dis. 2013;68:1-11.
3 Kaufmann C et al. Cornea. 2015;34(10):1326-1328.
4 Hong JP et al. Cornea. 2011;30(7):729-738.
5 Kim SW et al. Cornea. 2011;30(8):848-854.
6 Avellino: “New Avagen Genetic Test.” www.avellino.com/en/products/avagen-test.
7 Jun J et al. J Refract Surg. 2018;34(2):132-139.
8 Patel SV et al. Ophthalmology. 2020;127(3):315-323.
Meet the Experts
KATHRYN A. COLBY, MD, PHD Louis Block professor and chair of ophthalmology and visual science at the University of Chicago. Relevant financial disclosures: None.
DEBORAH S. JACOBS, MD Director of the ocular surface imaging center at Massachusetts Eye and Ear in Boston. She is also a part-time associate professor of ophthalmology at Harvard Medical School. Relevant financial disclosures: None.
EUNG KWEON KIM, MD, PHD Professor of ophthalmology at Yonsei University College of Medicine in Seoul, South Korea. Relevant financial disclosures: Avellino Lab, USA: C.
KEN K. NISCHAL, MD, FRCOPHTH Chief of pediatric ophthalmology, strabismus, and adult motility at the University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh and medical director of UPMC Children’s telemedicine program. Dr. Nischal is also director of pediatric program development at the UPMC Eye Center and professor of ophthalmology at UPMC in Pittsburgh. Relevant financial disclosures: None.
JAYNE S. WEISS, MD Chair of ophthalmology and professor of ophthalmology, pathology, and pharmacology at Louisiana State University (LSU) in New Orleans. She is also chief medical officer at LSU Healthcare Network, associate dean of clinical affairs at LSU Medical School, and director of LSU Eye Center of Excellence. Relevant financial disclosures: None.
Full Financial Disclosures
KATHRYN A. COLBY, MD, PHD GlaxoSmithKline: C; WL Gore: C.
DEBORAH S. JACOBS, MD Neuroptika: C; Novartis: C; Simple Contacts: C,O; TECLens: C,O.
EUNG KWEON KIM, MD, PHD Avellino Lab, USA: C.
KEN K. NISCHAL, MD, FRCOPHTH Carl Zeiss Meditech: S; Nevakar: C.
JAYNE S. WEISS, MD Horizon Therapeutics: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|