Download PDF
Researchers have developed a method for transforming fibroblasts into rod photoreceptors that, when implanted into blind mice, enabled the animals to detect light and exhibit visual responses.1 This novel technique skips the previously necessary step of first converting the fibroblasts into induced pluripotent stem cells (iPSCs), which then can be differentiated into retinal cells. That process takes up to six months to complete, compared to 10 days with the new method.
“Until now, no one has been able to convert fibroblasts directly to photoreceptors,” said study coauthor Anand Swaroop, PhD, at the NEI.
The NEI-funded researchers who developed the new cellular reprogramming technique were led by Sai H. Chavala, MD, at the University of North Texas Health Science Center School of Medicine in Fort Worth. Dr. Swaroop said that his laboratory at the NEI primarily contributed by performing the genetic analyses needed to validate that the new cells were expressing the proper photoreceptor genes.
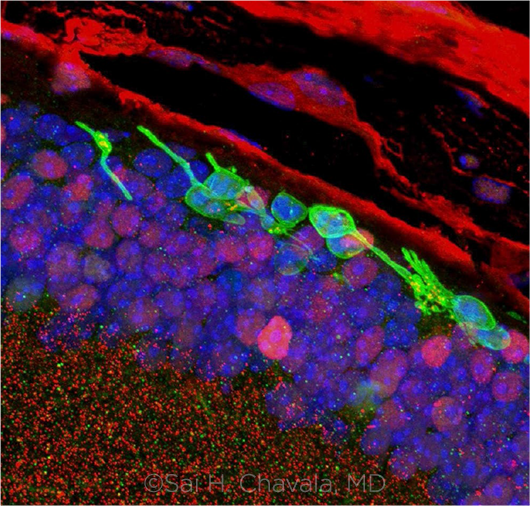 |
RESULTS. Three months after transplantation, immunofluorescence studies confirmed the survival of the chemically induced photoreceptor-like cells (green cells). They also show integration of the cells into the layers of the mouse retina.
|
A five-year quest. In a painstaking series of experiments that spanned five years, Dr. Chavala and his colleagues discovered that they could coax both mouse and human fibroblasts to become retinal cells by bathing them in a chemical cocktail of five small molecular compounds. These compounds were known individually to play a role in rod photoreceptor development.
When the transformed cells were transplanted into the subretinal space of mice that lacked rods, there were signs that the animals could detect light. Six of 14 mice (43%) had robust pupil constriction in low-light conditions, compared to none of the untreated controls. The mice with pupil constriction also were more likely than both the untreated mice and those with no constriction to seek out dark places, which is a natural behavior in sighted mice. Immunofluorescent images taken three months after transplantation showed that the cells were still viable and that their connections to neurons in the inner retina persisted.
What’s next? The University of North Texas has a patent pending on the methods reported in the paper. Dr. Chavala also is with CIRC Therapeutics, a spinoff company founded to conduct clinical trials and commercialize treatments using this cellular reprogramming method.
But Dr. Swaroop noted that much more research will be required in order to address two challenges: 1) how to increase the technique’s yield of functional cells, and 2) how to optimize their location and orientation in the retina, first in mice and eventually in humans.
A related finding. Dr. Swaroop said he also looks forward to learning more about the study’s most intriguing finding: that the chemical cocktail central to this technique activates mitochondria to produce reactive oxygen species (ROS) that are crucial to the cellular reprogramming. That is in contrast to the cell damage that ROS trigger in other ocular settings, he said.
“We don’t have the whole story yet. I think additional combinatorial mechanisms must be there,” he said. “I wonder how the mitochondrial reactive oxygen species activate [the cellular reprogramming processes] but do not go on to cell-damaging pathways. How are those other pathways inhibited? That part is still very intriguing and might have major implications broadly for regenerative medicine.”
—Linda Roach
___________________________
1 Mahato B et al. Nature. Published online April 15, 2020.
___________________________
Relevant financial disclosures—Dr. Swaroop: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Brooks None.
Dr. Milea Note: This study was funded by the Singapore National Medical Research Council (CS-IRG Grant) and the SingHealth Duke–NUS Ophthalmology and Visual Sciences Academic Clinical Program.
Dr. Swaroop None.
Dr. Walter Allergan: C; Castle Biosciences: C; Genentech: C.
Dr. Wong Allergan: C; Bayer: C; Boehringer-Ingelheim: C; EyRis: O; Genentech: C; Merck: C; Novartis: C; Oxurion: C; plano: O; Roche: C; Samsung: C. Note: This study was funded by the Singapore National Medical Research Council (CS-IRG Grant) and the SingHealth Duke–NUS Ophthalmology and Visual Sciences Academic Clinical Program.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review