By Gary N. Holland, MD, with Debra A. Goldstein, MD, James T. Rosenbaum, MD, and Russell N. Van Gelder, MD, PhD
Download PDF
In last month’s EyeNet, Gary N. Holland, MD, of the UCLA Stein Eye Institute, hosted a roundtable discussion on the workup for uveitis and other inflammatory eye diseases. In this second of 3 installments, Dr. Holland continues his conversation with Debra A. Goldstein, MD, of Northwestern University’s Feinberg School of Medicine; James T. Rosenbaum, MD, of Oregon Health & Science University’s Casey Eye Institute and the Legacy Devers Eye Institute; and Russell N. Van Gelder, MD, PhD, of the University of Washington. These experts share thoughts on the application of Bayes’ theorem to lab testing, human leukocyte antigen (HLA) typing, repeating lab analyses, and new imaging modalities. The final segment of this series, to appear next month, will cover issues related to ordering tests and give a look at future developments.
Bayes’ Theorem
Dr. Holland: Dr. Rosenbaum, you’ve written on the subject of Bayes’ theorem. I’d like to ask you about the implication of Bayes’ theorem when confronted with a positive result of a test that’s ordered for nearly every patient.
Dr. Rosenbaum: Thomas Bayes was an 18th-century British mathematician and minister who theorized that the interpretation of a probability test result must include knowledge of pretest probabilities to put the result in context. Let me clarify this with a practical example. A fluorescent treponemal antibody absorption (FTA-ABS) test for syphilis is approximately 99% sensitive and 99% specific. That is, for everyone who has syphilis, the test result is positive 99% of the time. For everyone who does not have syphilis, the result is negative 99% of the time. If you had 1,000 consecutive patients with uveitis, and you performed an FTA-ABS test on every patient, you would have 10 false positives. Therefore, your interpretation of the result would depend on how often syphilis occurs in your uveitis population. For many practices, approximately 1% of patients have syphilis. In that case, 10 of 1,000 consecutive patients would have syphilis. If you screened these patients for syphilis and uveitis, you’d have as many false positives as true positives. There would only be a 50/50 chance that a patient with a positive test result actually had syphilis. If you then got a positive VDRL (Venereal Disease Research Laboratory) result, that would nail it for you. However, for a patient with tertiary syphilis or late secondary syphilis with a negative VDRL result, you wouldn’t know whether it was a false positive or a true positive.
On the other hand, if you knew more about the form of uveitis (for example, it didn’t respond to prednisone), or if the patient had multiple sexual partners, you could estimate that perhaps the patient had a 50% likelihood of syphilis. If you then get a positive FTA-ABS result, your interpretation of the result would be different; you could be more confident that the patient has syphilis.
You can make the same sort of extrapolation about an antinuclear antibody (ANA) test for lupus or a Quantiferon or purified protein derivative (PPD) test for tuberculosis (TB). Knowledge of pretest probability should impact your interpretation of a test.
Dr. Holland: I think that’s an excellent point. Anytime you’re ordering a test frequently, you have to consider the possibility of false positives. When it comes to screening for syphilis or TB, I always will involve an infectious disease specialist for follow-up on a positive result. I’ve seen patients who were treated for syphilis who never actually had the disease but had a false-positive result.
Dr. Goldstein: That description of Bayes’ theorem helps explain why there isn’t a testing panel for uveitis; we need to test based on the clinical picture. If you test every person with any form of uveitis for HLA-B27, the chance of the result being positive but unrelated to the uveitis is quite high. However, if we limit our HLA-B27 testing to patients with acute anterior uveitis, the chance of a positive result being related to the uveitis becomes much higher.
Similarly, if you see a patient with an infiltrative retinitis, which is characteristic of syphilis, the chance of a positive syphilis result being unrelated to the retinitis is very small. If the patient instead had mild anterior uveitis, the uveitis likely would be unrelated to a positive syphilis result.
I think it’s important to recognize the difference between false positives and unrelated true positives. A positive Quantiferon or a positive PPD can be a true positive, indicating previous exposure to TB, without being related to the eye disease.
 |
|
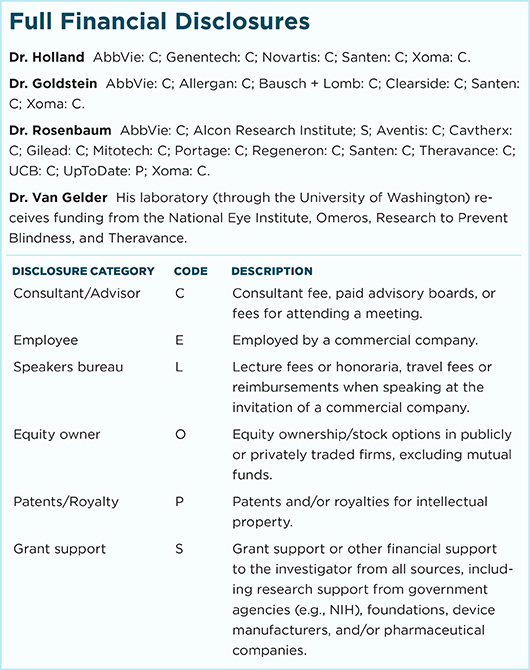
HLA-B27–ASSOCIATED UVEITIS. In patients with isolated sudden-onset unilateral acute anterior uveitis, HLA-B27 can be valuable. With positive test results, the physician can better set patient expectations about disease course and outcome. A positive HLA-B27 may also raise suspicion for spondyloarthropathy. (This image was originally published in the ASRS Retina Image Bank. Henry J. Kaplan, MD. HLA-B27 Associated Uveitis. Retina Image Bank. 2014; Image Number 18312.)
|
Positive Test Results
Dr. Holland: When you’re confronted with a positive test result for an investigation that you order frequently or that’s ordered by the referring physician, such as a serologic test for syphilis or a skin or blood test for TB, what do you actually do with that positive result?
Dr. Goldstein: A positive HLA-B27 result helps me guide the patient in terms of counseling for recurrences of their uveitis. If the patient had a completely negative review of systems for their joints, I would just discuss ankylosing spondylitis. If there are any joint symptoms, I would refer the patient to a rheumatologist, with a probable diagnosis of ankylosing spondylitis.
For TB testing, if I think that the uveitis could be caused by TB (which would be rare), I would involve an infectious disease specialist to treat the patient for active TB. For a TB-positive patient for whom I don’t suspect TB as a cause of the uveitis, I would want the latent TB to be treated before I start immunosuppression.
 |
|
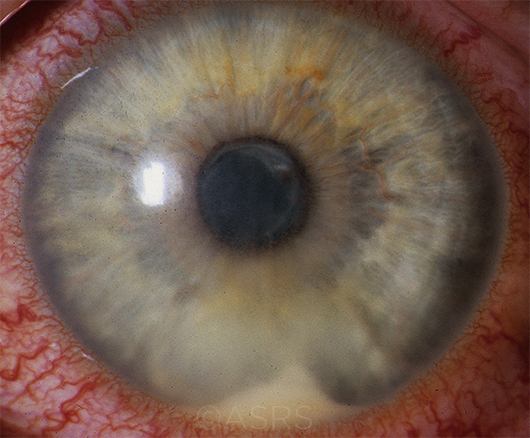
DIAGNOSIS UVEITIS? Birdshot chorioretinopathy (BSCR) occurs almost exclusively in HLA-A29–positive individuals. If a clinician is not certain about a diagnosis of BSCR based on history, symptoms, and clinical findings, a positive test for HLA-A29 can provide additional support for the diagnosis. However, a positive test alone never proves a diagnosis, as many HLA-A29–positive individuals never develop the disease. On the other hand, a negative test for HLA-A29 casts doubt on the diagnosis and should spur the clinician to consider other possibilities, such as intraocular lymphoma or ocular sarcoidosis. (This image was originally published in the ASRS Retina Image Bank. Jason S. Calhoun, Ophthalmic Photographer, Mayo Clinic, Jacksonville, Fla. Birdshot. Retina Image Bank. 2017. Image Number 7792.)
|
HLA Typing
Dr. Holland: Do you always order an HLA-B27 screen for someone who has unilateral acute anterior uveitis? What are your thoughts about its value?
Dr. Rosenbaum: I wouldn’t say I always order it, but frequently it is accurate. Approximately 7% of the U.S. population has the HLA-B27 allele, so with a screen, you will identify some HLA-B27–positive patients for whom there’s no relationship between that HLA type and the inflammation. However, if someone has an isolated, sudden onset of unilateral anterior uveitis with decreased intraocular pressure—or at least not elevated intraocular pressure—and without keratouveitis to suggest a viral cause, there’s a strong likelihood that HLA-B27 is playing a causal role. For these patients, there’s a reasonable chance that the uveitis will recur, so I would discuss management of recurrences with prompt use of topical corticosteroids followed by a return visit.
HLA-B27–positive patients also are very likely to have chronic back pain, tendonitis, or arthritis. Results of the DUET1 and SENTINEL2 studies confirmed that a huge percentage of patients with sudden-onset unilateral anterior uveitis and HLA-B27 positivity do have spondyloarthropathy. So I think HLA-B27 testing can be very helpful.
Dr. Goldstein: HLA-B27 testing can make a big difference for the patient. For many patients, I’m the one to diagnose probable ankylosing spondylitis. Undiagnosed patients may have undergone steroid injections or disc surgery for back pain. A few of my female patients had hysterectomies for what was diagnosed as pelvic pain but actually was ankylosing spondylitis. If a patient has chronic or intermittent low back pain and is HLA-B27 positive, they should see a rheumatologist—not an orthopedic surgeon or an Ob/Gyn—and get treatment for the arthritis.
Dr. Holland: The argument against HLA-B27 testing has been that the results won’t change your management of the patient, but I also order the test for nearly all patients who have findings suggestive of HLA-B27 disease. The results give the patient some prognostic information, including their risk for other inflammatory diseases.
Dr. Van Gelder: HLA-B27 testing provides good prognostic information about a patient’s uveitis. We have a large body of literature on what to expect with B27 acute anterior uveitis. We know that it is likely to recur. Even though the patient may present in extremis with reduced vision and severe discomfort, we know that it will be a limited disease process. We know that, in the long term, it is unlikely that there would be significant vision loss from this condition. We are more confident presenting that information if we know it’s an HLA-B27–associated disease, and I think the patient appreciates knowing the prognosis.
Dr. Holland: What other HLA tests are of value? When do you order an HLA test, and how do you interpret it?
Dr. Van Gelder: The only other HLA test that I order is HLA-A29 in the setting of birdshot chorioretinopathy. Nearly every case of birdshot that’s been described in the literature occurs in HLA-A29–positive patients. If a patient presents with what looks like birdshot but I’m not 100% sure, I would test for HLA-A29. If the result is negative, I’d be considerably more aggressive in looking at other possibilities for the disease, including lymphoma. I do not routinely get HLA-B51 or -B5 testing for Behçet disease. I think that the relative risk of disease is too low for those tests to be useful. One could argue for HLA-DR testing in tubulointerstitial nephritis with uveitis syndrome, but there are other tests for this condition that are very specific and sensitive, as well as cheaper and more direct.
Dr. Holland: I think an important point for our audience is that HLA test results can tell us about risk, but they should never be the sole basis for a diagnosis.
Dr. Rosenbaum: Also, the result is immutable. I’ve occasionally encountered a patient whose HLA typing has been repeated. Essentially, the HLA type on the day you’re born remains the same until the day you die.
Dr. Holland: The only exception may be when the patient’s test was performed long ago. Modern genetic tests are more accurate than previous antibody-based tests. If the clinician strongly suspects a particular HLA type, repeating the test once may put to rest any concern about older false-negative tests.
Repeat Lab Testing
Dr. Holland: Do you periodically repeat laboratory tests on patients for whom you’ve not established a causal diagnosis?
Dr. Goldstein: I repeat a review of systems, but I don’t see the value of repeating tests if the review of systems hasn’t changed and if the disease isn’t proceeding in an unexpected way such that my differential has changed.
If a patient with suspected sarcoidosis for whom I had no evidence of systemic disease suddenly mentions episodes of syncope, palpitations, or shortness of breath, I would be concerned that the patient has cardiac or pulmonary sarcoid. In that case, I would send the patient to the appropriate physician to work up those concerns. However, I don’t think that repeating lab testing periodically—absent a change in systemic or ocular disease—is a cost-effective or time-effective strategy.
Dr. Rosenbaum: How often is uveitis the initial manifestation, and months or years later, the systemic disease comes along? That happens, for example, in inflammatory bowel disease, but it’s not common. I agree that repeating a review of systems and historical questions is important. Sometimes I didn’t ask the right question when I first met the patient. Occasionally, it’s on the second, third, or fifth meeting that I realize an association with inflammatory bowel disease or even Behçet disease.
The only other setting where I might want to repeat a lab test is if the patient is not responding to conventional immunosuppression, which could suggest an infectious cause or a masquerade syndrome.
Dr. Holland: I agree. I only repeat diagnostic tests if the patient has a status change or presents with new information, including new physical signs or a change in the review of systems. I also repeat tests for patients being monitored, such as those on immunomodulatory therapies.
Dr. Van Gelder: When monitoring patients for drug toxicity, it always begins with history. You want to make sure you’re appropriately tailoring the medication to the patient. Someone who has a significant history of alcoholism or hepatitis is not a great candidate for methotrexate. Someone with renal disease or high blood pressure is not a great candidate for cyclosporine or tacrolimus.
My baseline exam typically includes a Quantiferon test to rule out TB, a complete blood count (CBC), and a comprehensive metabolic panel. I’m looking for underlying occult anemia or leukopenia as well as underlying electrolyte, liver function, or renal abnormalities. This is a critical baseline because these tests may influence the clinician’s choice between treatment options that have different systemic toxicities and because the tests are going to be monitored throughout treatment for changes from baseline values.
Different drugs have different protocols. If you’re starting someone on Cytoxan (cyclophosphamide), your attention to their white blood cell count will be much higher than if you’re starting them on methotrexate. Initially, I would follow patients starting Cytoxan weekly with CBC testing. For patients on most of the antimetabolites and anti-TNFs (tumor necrosis factor drugs), I typically retest at 1 month for idiopathic acute liver or kidney effects and at 3-month intervals thereafter.
There’s not much data regarding optimal testing regimens, and I’m not sure that we do this in the most cost-effective fashion because intolerance at the level of laboratory testing is relatively rare. In the SITE study,3 laboratory intolerance rates were approximately 20%, so there’s a fair chance with methotrexate, for example, of finding liver function abnormalities. That said, it is worth targeting testing to the immunomodulators being utilized—for instance, testing liver functions for methotrexate or renal function for cyclosporine.
Dr. Rosenbaum: For most of the anti-metabolites, I get a baseline CBC with differential and a complete metabolic panel that includes liver function, and I repeat after 1 month. If everything’s okay, I repeat testing every 2 months indefinitely. If you’re going to prescribe azathioprine, some experts recommend prior testing for thiopurine methyl transferase, which is expensive and time-consuming. I instead start with a low dose of azathioprine. I don’t test for TB exposure when I use an antimetabolite, but I always test for TB exposure if I’m going to use a biologic, such as a TNF inhibitor. Blood pressure monitoring is very important if you’re going to use a calcineurin antagonist like cyclosporine.
New Imaging Modalities
Dr. Holland: There are several newer imaging modalities, including widefield photography and angiography with the Optos camera, optical coherence tomography angiography, near-infrared reflectance, and fundus autofluorescence. We have relatively little experience with these modalities in patients with uveitis, so how do you manage a positive finding, such as peripheral vascular leakage, in an otherwise quiet eye?
Dr. Goldstein: In the past, we didn’t have the ability to look in the far periphery with angiography. I think we’re going to find that even many patients with anterior uveitis and an inflamed ciliary body will have peripheral vascular leakage. If the vasculitis is not involving the posterior pole, the vascular leakage is only in the periphery, and the eye is otherwise quiet, I would observe rather than treat. I’ve had patients come to me on very aggressive immunosuppression for only far-peripheral vascular leakage, and I don’t think we have any data to support a need for such treatment.
Dr. Rosenbaum: When we started doing echocardiography, we found that many people had mitral valve prolapse, and when we started doing magnetic resonance imaging of the brain, we found that many people had unidentified bright objects. If we look hard enough, I think we’ll see a lot of peripheral retinal vascular leakage that’s not clinically significant.
Dr. Van Gelder: I agree with respect to phlebitis. We really don’t know what the long-term implications are for peripheral, particularly venous, leakage. On the flip side, sometimes you will see things with these modalities that are treatable. When I see significant nonperfusion on the arterial side, it indicates an inflammatory cause of disease that we need to treat more aggressively.
Dr. Holland: I think we all agree that for each new technique, information from a large number of patients needs to be collected and evaluated before we understand the implications of a test finding from any individual patient.
___________________________
Listen to the roundtable below:
___________________________
1 Haroon M et al. Ann Rheum Dis. 2015;74(11):1990-1995.
2 Juanola X et al. Ophthalmology. 2016;123(8):1632-1636.
3 Gangaputra S et al. Ophthalmology. 2009;116(11):2188-2198.
___________________________
Dr. Holland is Professor of Ophthalmology and Jack H. Skirball Chair in Ocular Inflammatory Diseases, David Geffen School of Medicine at University of California, Los Angeles, UCLA Stein Eye Institute. Relevant financial disclosures: None.
Dr. Goldstein is Magerstadt Professor of Ophthalmology at Northwestern University Feinberg School of Medicine in Chicago. Relevant financial disclosures: None.
Dr. Rosenbaum is Professor of Ophthalmology, Medicine, and Cell Biology; Head of the Division of Arthritis and Rheumatic Diseases; and Edward E Rosenbaum Professor of Inflammation Research at Oregon Health & Science University’s Casey Eye Institute in Portland. He is also the Richard Chenoweth Chair of Ophthalmology at Legacy Devers Eye Institute in Portland. Relevant financial disclosures: UpToDate: P.
Dr. Van Gelder is Boyd K. Bucey Professor and Chair of Ophthalmology at the University of Washington in Seattle. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.