By Rebecca Taylor, Contributing Writer, interviewing Chiu M. Gemmy Cheung, MBBS, FRCOphth, Gregg T. Kokame, MD, MMM, and Timothy Y.Y. Lai, MD, FRCOphth
Download PDF
Greater awareness of the need to diagnose polypoidal choroidal vasculopathy (PCV) begs the question of best practices for treatment. Do therapies used for other forms of age-related macular degeneration (AMD) work? And do therapies work for both components of PCV—its characteristic polypoidal lesions and its branching neovascular networks—to dry out the eye and occlude the lesion itself?
Traditionally, clinicians have been able to visualize the polypoidal lesion much better than the branching neo-vascular network when using indocyanine green angiography (ICGA), said Chiu M. Gemmy Cheung, MBBS, FRCOphth, at Duke-NUS Medical School in Singapore. This has led to the use of an occlusive method to target the lesion with focal laser or photodynamic therapy (PDT), she said.
But now, “with the advent of anti-VEGF therapy for typical neovascularization, anti-VEGF is being used in PCV,” Dr. Cheung said. “Using OCT as an assessment tool, we have observed that anti-VEGF can reduce exudation effectively, although the rate of occluding the polypoidal lesions may be lower than therapies that include laser.”
 |
|
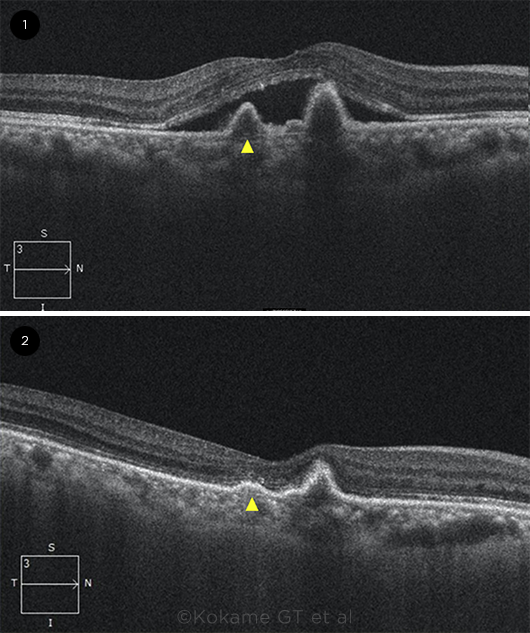
ANTI-VEGF IMPACT. OCT B-scan images (1) before and (2) after anti-VEGF treatment. Arrowhead = a U-shaped elevation of the retinal pigment epithelium that is typical of polypoidal lesions.
|
A Growing Role for Anti-VEGF
Last year, the Asia Pacific Ocular Imaging Society (APOIS) clarified its latest thinking on how the pathophysiology of PCV differs from typical AMD.1
“Our newest understanding is that the neovascularization is the backbone of the PCV complex,” said Dr. Cheung. “We treat the exudation with anti-VEGF, whereas the polypoidal lesions behave like a complication of the network that can come and go.”
“With this type of choroidal neovascularization, the blood vessels develop a bulge at the end of the vessels, or within the vascular complex, so the polypoidal lesions are actually part of the abnormal subretinal vessels themselves,” added Gregg T. Kokame, MD, MMM, at the University of Hawaii in Honolulu.
The big three. Bevacizumab (Avastin), ranibizumab (Lucentis), and aflibercept (Eylea) are all used to treat PCV. “I would be happy to use any of the three commonly available anti-VEGF agents,” said Dr. Cheung. She added, “I start off with three monthly loading injections and, after achieving a dry retina, continue with a treat-and-extend protocol. If there is persistent fluid after the three initial doses, I might add three further monthly injections until month 6.”
“We’ve been using ranibizumab and bevacizumab over the past 15 years to treat PCV, and aflibercept in the 10 years it’s been available,” said Timothy Y.Y. Lai, MD, FRCOphth, at the Chinese University of Hong Kong.
“Ranibizumab works well as a monotherapy: It can maintain or improve vision, and even reduce some polypoidal lesions, but frequent injections are required,” Dr. Lai said. “Some data show that aflibercept might be slightly better compared with ranibizumab, but there is no real head-to-head trial, so it’s extrapolated data.”
What about brolucizumab? The newest anti-VEGF drug, brolucizumab (Beovu), was approved in 2019 but is not yet available worldwide.
In the HAWK and HARRIER trials, researchers compared brolucizumab to aflibercept. While the trials were designed for neovascular AMD, a later subset analysis of data from HAWK involving outcomes in patients with PCV showed comparable gains in vision with the two drugs and better resolution of intraretinal and subretinal fluid with brolucizumab than with aflibercept.2 Results of this analysis showed that brolucizumab is “the best agent to decrease subretinal fluid, but patients can develop severe vitritis and vasculitis with vision loss, so it’s only used when other agents have not had an adequate response, and patients are well informed of the risks of vision-threatening inflammation,” said Dr. Kokame.
The evidence evolves. The EVEREST II study compared ranibizumab monotherapy to ranibizumab plus PDT for treatment of PCV.3 And the PLANET study of PCV patients compared aflibercept monotherapy to aflibercept plus PDT—but PDT was only given as rescue therapy after 12 weeks.4
In PLANET, only 18% of patients met the criteria for rescue PDT, and most of these patients only received one PDT treatment. “Because of this design, the study really compares a group where 100% of patients were treated only with aflibercept to a group where 82% of patients were treated only with aflibercept, so the assessment of the effect of PDT was limited,” said Dr. Kokame.
“The PLANET data suggest we can be quite confident of using aflibercept monotherapy in about 80% of cases,” said Dr. Cheung. As for closing the polypoidal lesions, “the EVEREST II study with ranibizumab monotherapy achieved closure in about one in three lesions—and in the PLANET study, aflibercept monotherapy was similar, at 30% to 40%,” she said.
Ad hoc studies. Given the lack of large PCV trials, some retina specialists are doing their own research. “We did a number of studies on our own showing that patients receiving ranibizumab didn’t do as well as patients with aflibercept; they had more lesions and persistent leakage,” said Dr. Kokame. “When we switched to aflibercept, the lesions and leakage lessened. Bevacizumab also doesn’t work as well, anatomically, as aflibercept.”
And Dr. Cheung said, “In one of our latest trials in Singapore, we used aflibercept monotherapy to achieve polypoidal lesion closure by extending the loading phase to up to six doses in the first 24 weeks, and we achieved closure in 55% of the eyes.”5
A note on treatment response. The key with using anti-VEGF for PCV is to assess treatment response at month 3, looking at pigment epithelial detachments (PEDs) as well as vision and fluid, said Dr. Cheung.
“We have learned from the latest data that instead of the strict protocol to achieve a completely dry retina, we may be able to tolerate a little subretinal fluid if there are no other signs of disease,” said Dr. Cheung. However, “an aggressive polypoidal lesion may manifest as high PEDs with sub–retinal pigment epithelium rings, and these eyes may develop a sudden-onset hemorrhage and devastating reduction in vision.” Given this, she said, “I may recommend combination therapy to achieve control of disease faster.”
A note on insurance requirements. In the United States, the insurance companies play an outsized role in anti-VEGF protocols. As Dr. Kokame noted, “We often have to start with bevacizumab and are usually required to use at least three injections. Only then can we request alternative injections if there is anti-VEGF resistance.”
High Index of Suspicion Needed
With PCV, the risks of delayed diagnosis or treatment are high, the experts emphasized. The condition can lead to “gradual leakage in the macula with recurrent episodes of bleeding that can cause fibrosis—or one big episode of bleeding,” Dr. Lai said. “Breakthrough vitreous hemorrhage and hemorrhagic retinal detachment have also been reported, requiring vitrectomy to deal with these very complicated cases.”
With neovascular AMD, Dr. Kokame said, “injections of anti-VEGF medications are our usual treatment, but we often have cases that don’t respond—so it’s important to make the PCV diagnosis” in these instances.
Quiescence isn’t cure. PCV is now seen as a chronic disease requiring long-term follow-up, said Dr. Cheung. “We’ve come to realize it’s not just about giving three or six injections,” she said. “Many of these eyes will have recurrences after a period of quiescence lasting a few months, but it’s dangerous to wait until patients present with another drop in vision, because sometimes you can’t recover that vision again.”
“If you treat patients properly with anti-VEGF or combination therapy, they usually get a vision improvement in the first year or two, but they are very prone to recurrences in the future,” Dr. Lai said.
|
A Continuing Role for Combination Tx
A poor response to anti-VEGF injections is common with PCV, Drs. Cheung, Kokame, and Lai pointed out.
As Dr. Kokame noted, “PCV predicts anti-VEGF resistance, which is why it’s critical to make the diagnosis—and to look for PCV with OCT-B scan, en face OCT, and ICGA if available.” (For more on diagnosis, see part 1 of this story in the January issue.) He added that a PCV diagnosis “can suggest alternative therapy, including combination therapy with PDT or laser photocoagulation for extrafoveal polypoidal lesions.”
Use of PDT. Photodynamic therapy “is not performed enough in the United States, although PDT with anti-VEGF is a primary treatment we offer [PCV] patients, resulting in fewer injections and better anatomic results,” said Dr. Kokame. “In EVEREST II, it gave better vision with fewer injections.”
Specifically, EVEREST II found that ranibizumab plus PDT brought higher gains in visual acuity and nearly twice the rate of lesion closure (69.3% vs. 34.7%) compared to ranibizumab alone.3 These gains held at 24 months, and combination therapy required half the number of injections. Moreover, Dr. Lai noted, “In EVEREST II, 20% to 25% of patients after combination therapy required no additional treatment.”
Challenge of cost and access. Given the EVEREST II results, why not start with PDT? As Dr. Cheung explained, PDT is expensive—and it isn’t widely available. “Combination therapy requires additional setup, generally with ICGA, to target the lesion, a special laser, and the drug verteporfin,” she said. “Currently, there’s a global shortage of verteporfin.”
Fortunately, new research suggests that OCT-B scans can successfully target the PDT treatment sites, bypassing the need for ICGA. As the APOIS review reported, OCT-guided treatment spots were able to cover 90% of the branching neovascular networks and 100% of polypoidal lesions targeted by ICGA.1
On the Horizon: Future Tx
Bispecific antibodies, in-dwelling drug delivery, and gene therapy are in the running as future PCV treatments. “Agents promising increased durability have shown potential in neovascular AMD,” said Dr. Cheung. “We hope they will also benefit eyes with PCV.”
Faricimab. This investigational anti-VEGF drug, developed by Roche, targets two pathways. “Faricimab is a bispecific antibody that binds to angiopoietin-2 as well as VEGF,” said Dr. Lai. “It showed better durability in treatment effect in the TENAYA and LUCERNE studies on neovascular AMD, and, compared to brolucizumab, it was very well tolerated with few cases of intraocular inflammation.” PCV patients were recruited to both studies.
Reservoir drugs. With Susvimo (formerly known as the Port DeliverySystem), “you put a reservoir of ranibizumab inside the eye in the pars plana that lasts six to nine months, with the ability to refill the reservoir with an office procedure,” said Dr. Kokame.
In-dwelling drug delivery addresses the goal of treatment durability. Susvimo (Roche) is now FDA approved for treatment of neovascular AMD. “Some PCV patients require such frequent injections—and with this device, you might be able to keep the macula at the quiescent stage,” said Dr. Lai.
Gene therapy. Gene therapy targets the ARMS2 and HTRA1 genes associated with AMD. Studies involve injecting a virus vector inside the eye, which induces the eye to produce its own anti-VEGF treatment, Dr. Kokame said.
As for delivery, “Gene therapy is being developed with subretinal, intravitreal, and suprachoroidal delivery methods,” he said.
___________________________
1 Teo KYC et al. Ophthalmol Retina. 2021;5(10):945-953.
2 Ogura Y et al. Br J Ophthalmol. Published online July 22, 2021.
3 Lim TH et al. JAMA Ophthalmol. 2020;138(9):935-942.
4 Chaikitmongkol V et al. Asia Pac J Ophthalmol. 2020;9:260-268.
5 Teo KYC et al. Br J Ophthalmol. Published online Feb. 11, 2021.
___________________________
Dr. Cheung is professor of ophthalmology and visual sciences at Duke-NUS Medical School in Singapore and head and senior consultant of the Medical Retina Department at the Singapore National Eye Centre. Relevant financial disclosures: Allergan: C,L,S; Avirmax: C,L,S; Bayer: C,L,S; Boeringher Ingelheim: C,L,S; Carl Zeiss: C,L,S; Novartis: C,L,S; Roche: C,L,S; Topcon: C,L,S.
Dr. Kokame is chief of ophthalmology and clinical professor at the University of Hawaii School of Medicine, medical director of the Hawaii Macula and Retina Institute, and founding partner and senior consultant of Retina Consultants of Hawaii, all in Honolulu. Relevant financial disclosures: Bausch + Lomb: C,L; Carl Zeiss: L; Genentech: S; Novartis: S; Regeneron: S; RegenxBio: S.
Dr. Lai is a clinical professor (honorary) of ophthalmology and visual sciences at the Chinese University of Hong Kong. Relevant financial disclosures: Allergan: C,L,S; Bayer: C,L,S; Boeringher Ingelheim: C,L,S; Novartis: C,L,S; Roche: C,L,S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Cheung Allergan: C,L,S; Avirmax: C,L,S; Bayer: C,L,S; Boeringher Ingelheim: C,L,S; Carl Zeiss: C,L,S; Novartis: C,L,S; Roche: C,L,S; Topcon: C,L,S.
Dr. Kokame Bausch + Lomb: C,L; Bayer: C; Carl Zeiss: L; Genentech: S; Iveric: S; Novartis: S; Regeneron: S; Roche: C; RegnxBio: S; Salutaris: S.
Dr. Lai Allergan: C,L,S; Bayer: C,L,S; Boeringher Ingelheim: C,L,S; Novartis: C,L,S; Roche: C,L,S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|