Download PDF
In the ideal world of controlled scientific studies, the strategy of treating proliferative diabetic retinopathy (PDR) with intravitreal injections has proved effective and possibly superior to panretinal photocoagulation (PRP). Now researchers report that in the “real” world, where patients are often lost to follow-up, PRP may be the better option.1
“Part of the impetus for this study was that we know PRP has long-lasting effects on stabilizing PDR, but we have little data on whether anti-VEGF therapy has any long-lasting effects once it is stopped,” said Jason Hsu, MD, at Wills Eye Hospital in Baltimore.
Now, data exist. “Our study suggests that if there is a period of loss to follow-up, patients with PDR who receive PRP may have better outcomes compared to those who received only anti-VEGF therapy,” said coauthor Anthony Obeid, MD, MPH, also at Wills Eye Hospital.
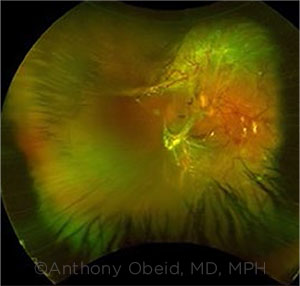 |
RD RISK. This eye with PDR developed an RD after being lost to follow-up.
|
Retrospective cohort. The findings are based on medical records of 59 patients with PDR (76 eyes) who returned at various time points for follow-up treatment. All of the 59 patients had been lost to follow-up for 6 or more months immediately after receiving either intravitreal injections (20 patients; 30 eyes) or PRP (39 patients; 46 eyes).
Findings include the following:
- Visual acuity (VA) scores worsened in anti-VEGF eyes, from 20/54 at the visit before patients were lost to follow-up to 20/187 at the return visit and 20/ 166 at the final visit.
- In PRP eyes, VA significantly worsened from 20/53 at the visit before patients were lost to follow-up to 20/83 at the return visit. However, VA improved by the final visit to 20/58.
- There was a significantly greater incidence of neovascularization of the iris in the anti-VEGF group compared to the PRP arm at the final visit (4 vs. 0).
- A significantly greater number of eyes in the anti-VEGF group had tractional retinal detachment (RD) after patients returned to care. At the return visit, 5 in the anti-VEGF group experienced tractional RD, versus none in the PRP group. At the final visit, 10 anti-VEGF patients had tractional RD, versus 1 PRP patient. However, the incidence of tractional RD was lower in eyes that received a greater number of anti-VEGF injections prior to being lost to follow-up. “This may suggest that receiving a certain minimum number of injections may have lasting effects on PDR regression,” Dr. Obeid said.
Stick with PRP. The findings are particularly relevant as practice patterns are shifting toward anti-VEGF monotherapy for eyes with PDR, the authors said. They assume even greater relevance given the “strikingly high” rates of patients lost to follow-up,2 said Dr. Hsu.
“Some clinicians believe that PRP may not be necessary or can be delayed while the patient is actively receiving anti-VEGF treatments,” Dr. Hsu added. “However, our study suggests that physicians may want to proceed with PRP at an earlier time point given the potential for poorer outcomes with erratic follow-up.” (For more on this topic, see “Five-Year Outcomes of Randomized Trial Comparing Laser with Ranibizumab for PDR” in this month’s Journal Highlights.)
—Miriam Karmel
___________________________
1 Obeid A et al. Ophthalmology. Published online Aug. 2, 2018.
2 Obeid A et al. Ophthalmology. 2018;129(9):1386-1392.
___________________________
Relevant financial disclosures—Drs. Hsu and Obeid: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Beltran Foundation Fighting Blindness: S; NIH/NEI: S; Research to Prevent Blindness: S; The Shaler Richardson Professorship Endowmment: S; University of Pennsylvania: P. Dr. Lewin: NEI: S; University of Florida: P.
Dr. Grand None.
Dr. Grönlund Agreement Concerning the Research and Education of Doctors (Gothenburg): S; Bayer: L; Cronqvists Foundation: S; De Blindas Vänner: S; Gothenburg Medical Society: S; Swedish Society of Medicine: S.
Dr. Hsu Roche/Genentech: S; Ophthotech: C,S; Santen: S.
Dr. Lewis NEI: S; University of Florida: P.
Dr. Obeid None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review