Download PDF
How often do patient safety issues occur with intravitreal injections in your practice? Researchers at the Mayo Clinic in Rochester, Minnesota, set out to determine the root cause of injection-related safety events in their high-volume clinic—and they developed a plan that significantly reduced the risk of these medical errors.1
“It’s easy to assume errors do not occur in your own practice,” said Sanjay V. Patel, MD, FRCOphth, lead author of the study. “But the frequency of events in a practice is probably underestimated until they are actually tracked in a transparent manner.”
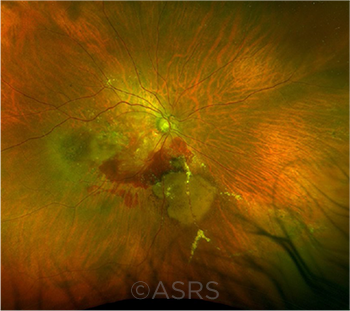 |
RISK REDUCTION. Intravitreal injections for conditions such as neovascular age-related macular degeneration (shown here) have become a common ophthalmic procedure. Site marking and dual verification can reduce the risk of injection-related medical errors. This image was originally published in the ASRS Retina Image Bank. Ambar Faridi, MD, and Vicki Moore. Neovascular Age-Related Macular Degeneration (1). 2021; Image Number 74742. © The American Society of Retina Specialists.
|
Tracking errors. For three years, the researchers tracked events in the clinic’s database that were documented as “never events” (i.e., medical errors that are clearly identifiable, preventable, and serious) or “near misses.” The former included wrong eye, wrong medication, and wrong patient scenarios. The latter occurrences might have resulted in a never event if they had been missed.
Creating a plan. A safety plan was implemented based on the findings. The plan reflected the Mayo Clinic’s model, in which the injecting physician usually is not the prescribing physician, and injections and outpatient retina care are delivered in separate settings.
The plan called for the following: 1) standardized documentation to reduce ambiguity and simplify interpretation of the treatment plan; 2) scheduled time for injecting physicians to review the prescribing physician’s treatment plans; and 3) a designated nurse or technician to verify treatment plans and serve as liaison between the retina and injection clinics. The plan also required wristbands to be affixed prior to patient handover for injection.
Before and after. At baseline, the rate of all events was 0.1% (28 of 27,400 intravitreal injections; 9.3 events per year). Three were never events that involved the wrong eye; the remaining 25 were near misses related to laterality (n = 11), medication (n = 10), and timing of injection (n = 4). No events were associated with patient harm.
After plan implementation, the rate of all events dropped to 0.01% (1 of 9,375 intravitreal injections). While the baseline frequency may appear low, it amounted to almost one event per year, said Dr. Patel. Although most of these events were near misses, Dr. Patel noted that low frequency events can still translate into high absolute numbers for high volume procedures.
Safety tips. Dr. Patel acknowledged that most practices administer injections as they see patients in outpatient clinics. Thus, there may be minimal gap between decision-making and the procedure. Still, he said, every practice can require site marking as well as dual verification of the treatment plan. This is especially true if there’s a gap, no matter how small, between the physician making the treatment decision and the one performing the procedure, he said.
At a minimum, Dr. Patel advised simply addressing the topic. “All practices should discuss the potential for errors with injections to raise awareness and promote a culture of safety.”
—Miriam Karmel
___________________________
1 Patel SV et al. Ophthalmol Retina. Published online Feb. 10, 2022.
___________________________
Relevant financial disclosures: Dr. Patel—None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Acharya NEI: S.
Dr. Ganguly None.
Dr. Lin None.
Dr. Patel AbbVie: C; Aerie: P; Emmecell: C; GlaxoSmithKline: C; Iris Medicine: P.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Hired to work for compensation or received a W2 from a company. |
| Employee, executive role |
EE |
Hired to work in an executive role for compensation or received a W2 from a company. |
| Owner of company |
EO |
Ownership or controlling interest in a company, other than stock. |
| Independent contractor |
I |
Contracted work, including contracted research. |
| Lecture fees/Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Patents/Royalty |
P |
Beneficiary of patents and/or royalties for intellectual property. |
| Equity/Stock/Stock options holder, private corporation |
PS |
Equity ownership, stock and/or stock options in privately owned firms, excluding mutual funds. |
| Grant support |
S |
Grant support or other financial support from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and\or pharmaceutical companies. Research funding should be disclosed by the principal or named investigator even if your institution receives the grant and manages the funds. |
| Equity/Stock/Stock options holder, public corporation |
US |
Equity ownership, stock and/or stock options in publicly traded firms, excluding mutual funds. |
More from this month’s News in Review
|