By Jing Sun, BA; Robert A Sisk, MD; Elias I. Traboulsi, MD, MEd; Virginia Miraldi Utz, MD
A Compendium of Inherited Disorders and the Eye, Oxford University Press
OMIM Number
Introduction
Norrie disease (ND) is an extremely rare X-linked recessive disease caused by pathogenic NDP mutations that result in defective norrin. Norrin normally activates the Wnt canonical cascade responsible for angiogenesis and cell specialization in the retina and vascular preservation in the inner ear. Patients with ND present with blindness due to leukocoria caused by immature retinal cell masses (pseudogliomas) and retinal detachments at birth. Most males develop sensorineural hearing loss during adolescence, and over a third of patients also present with cognitive disability, behavioral abnormalities such as autism, and/or psychotic-like features. Treatment strategies include early laser photocoagulation or vitrectomy to prevent retinal detachment. Patient with ND and hearing loss who undergo cochlear implants in adulthood report significant improvement to quality of life.
Nomenclature
Previous names for Norrie Disease:
- Episkopi blindness
- Pseudoglioma
- Fetal iritis blindness
- Anderson-Warburg Syndrome
- Norrie-Warburg Syndrome
- Whitnall-Norman Syndrome
Norrie Disease is part of the NDP-related retinopathies disease family (see “NDP-Related Retinopathies” below).
Inheritance
- X-linked recessive with near 100% penetrance in affected males.1,2
Gene/Gene Map
- Norrie disease protein gene (NDP): Norrin protein.
Xp11.32
- NDP is located on Xp11.3 and spans 28 kb of genomic DNA. The cDNA includes 3 exons, and most pathogenic variants are in the 3rd exon that encodes a cysteine-rich knot motif important for secondary and tertiary protein structure.3,4 Over 100 pathologic variants of NDP have been associated with Norrie Disease (ND)5 including missense, null, splice-site, and deletion mutations. Occasionally, a contiguous gene deletion (ie, deletions that could extend to neighboring genes) will occur, leading to a complex phenotype.5 Neighboring genes most likely to be involved in contiguous deletions are MAO-A, MAO-B, and EFHC2.2
Involved Molecular Pathways
NDP encodes the protein norrin, a ligand for the receptor frizzled-46, that activates the Wnt pathway to contribute to cell development and specialization (Figure 1).
Specifically, norrin has been shown to be involved in angiogenesis of small vessels of the retina and preservation of vascular structures in the inner ear.7-10 It is believed that pathogenic mutations alter the ability of norrin to bind frizzled-4, leading to decreased downstream activation of the Wnt cascade.3,4
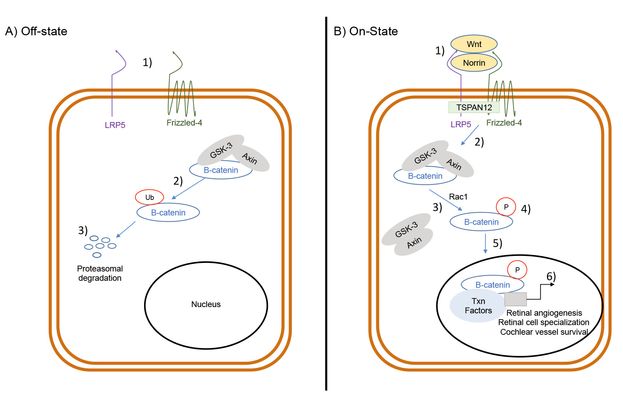
Figure 1. Wnt-signaling pathway
A) Off-state. No norrin activation of the receptor frizzled-4 (FZD4) (1) allows B-catenin to be ubiquitinated (2). B-catenin then undergoes proteasomal degradation (3) without being able to reach to nucleus to bind/recruit transcription factors.
B) On-state. Norrin and Wnt bind FZD4 (1). Through the help of LRP5 (2), FZD4 activates downstream proteins that disassociate GSK-3 and APC/Axin from B-catenin (3). This disassociation allows B-catenin to be phosphorylated (4) and stabilized before transportation into the nucleus (5). There, it binds with other transcription factors to regulate the transcription of mRNA (6) that promote retinal angiogenesis, retinal cell specialization, and vessel survival in the inner ear.
Epidemiology
- Currently, there is no estimate for total cases or annual incidence, as ND is exceedingly rare. There are no known racial, ethnic, or geographic correlations. ND has been documented in the USA, Europe, and Asia.2
- Although ND is a X-linked recessive disease, there have been rare reports of female carriers of NDP mutations who present with mild retinal abnormalities and mild hearing defects.11,12
Extraocular Clinical Findings
Overview
Currently, there are no official ND diagnostic criteria. Furthermore, variable expressivity and phenotypic variability are present among families sharing the same mutation.13
Male patients with classic ND present with 3 cardinal features: infantile blindness usually at birth or during the neonatal period, progressive hearing loss that begins in adolescence, and cognitive/behavioral issues that persist through life.1,5 Ocular manifestations are uniformly present and are the first sign of disease.2
To date, ND has not been proven to decrease overall life span.5
Auditory Findings
Norrin has been shown to prevent blood vessel degeneration in the inner ear of mice.8 Thus, NDP mutations and defective norrin may cause progressive loss of vessels in the stria vascularis of the cochlea and lead to insidious sensorineural hearing loss.2,8
Sensorineural hearing loss was originally reported in over 30% of ND patients;14 however, in more recent studies, it appears 100% of ND patients will eventually suffer some degree of hearing loss.5,15
Most patients develop auditory difficulties in early adolescence (10-14 years old), with over 85% of patients experiencing some degree of hearing loss by 25 years of life.5,15 Early symptoms were commonly described as intermittent “stuffiness” with continuous tinnitus.5 Symptoms progressively worsen into severe, bilateral deafness in adulthood.2 Unfortunately for ND patients, hearing loss appears to be one the biggest factors in quality of life with a large percentage reporting depression during the onset of their hearing loss.5 In a retrospective clinical questionnaire of 4 men with ND with successful cochlear implants, significant improvement in quality of life was reported for all four.5
Neurological Findings
The exact mechanism by which norrin influences the brain is still unclear, but in mouse models, norrin is seen in the forebrain, midbrain, and cerebellum.16 Although it has been proposed that norrin may affect brain vasculature, that hypothesis has yet to be supported, as brain MRIs of ND patients are usually normal.17
Cognitive and behavioral issues are identified in approximately 30%-50% of ND patients.2,5 It has been noted that a higher percentage of patients with potentially contiguous deletions versus intragenic deletions suffer from cognitive/behavioral issues.5 In the 2012 study by Smith et al, over 25% of patients reported autism/autism-like symptoms (14 of 51) and emotional lability (13 of 51).5
More recently, studies have begun to investigate seizures in ND patients. Although seizure disorders are not considered part of the classical ND presentation, Smith et al reported that 16% of their cohort had a history of seizures or seizure disorder.5 To date, there have been approximately 20 ND patients identified with seizures, but there are no consistent findings among these patients in terms of type of NDP deletion, seizure type, or EEG findings.17
Peripheral Vascular Disease (PVD)
The exact mechanism of NDP mutations and loss of norrin on PVD is still unknown. A few case studies of ND patients with vascular abnormalities have been reported in the recent literature, but there has only been one large-scale investigation of PVD in ND patients. In this study, 38% of ND patients reported issues with varicose veins, venous stasis ulcers, and/or erectile dysfunction (ED).5 In patients who reported ED, a small subset underwent angiography and were found to have decreased penile blood flow.5 Thus, more research is needed to determine the relationship of norrin to peripheral vascular disease.
Ocular Findings and Prognosis
Ocular manifestations by frequency and age are presented in Tables 1 and 2, respectively.
|
Table 1. Ocular manifestations of Norrie Disease by frequency18
|
|
Common
|
Frequent
|
Rare
|
|
Infantile blindness
Leukocoria
Abnormal retinal vasculature
Cataracts
Iris hypoplasia and degeneration
Microphthalmia
|
Nystagmus
Lens hypoplasia or aplasia
Retinal detachment
Hyaloidal vessel remnant
|
Pupil abnormalities
Ectopia lentis
Glaucoma
|
|
Table 2. Common Ocular Manifestations of ND by Age2
|
|
Birth/Neonatal Period
|
Infancy
|
Childhood
|
End-stage
|
|
Leukocoria
Retinal pseudoglioma
Infantile blindness
Retinal detachment
Retrolental fibrovascular proliferation
|
Cataracts
Anterior and posterior synechiae
Glaucoma
|
Corneal opacification
Band keratopathy
Hypotony (pre-phthisis)
|
Corneal opacification
Phthisis bulbi
|
Mechanism
In the normal retina, norrin activates the Wnt cascade to contribute to retinal angiogenesis and retinal cell specialization (Figure 1).16 Norrin has also been hypothesized to have neuroprotective properties on retinal ganglion cells.19,20 NDP mutations lead to defective or absent norrin production that results in abnormal retinal vasculature and formation of immature retinal cells.
Initial Presentation
Patients with classic ND present with complete bilateral retinal detachment and congenital blindness at birth.2 Of note, some patients develop retinal detachments several months after birth.2 During neonatal examinations, leukocoria secondary to pseudogliomas of the retina is commonly observed.2 Pseudogliomas are greyish-yellow masses that consist of fibrovascular material behind the lens that disrupts the normal red reflex.1,2 As the disease progresses, thick retrolental fibrovascular proliferation may develop and cause traction that may cause or worsen retinal detachment.21,22
Progression of disease
Through early infancy and childhood, patients continue to have ocular issues including cataracts, anterior and posterior synechiae, and angle closure.2 Anomalies of the anterior chamber may lead to outflow tract disruptions and result in glaucoma and discomfort/pain.2
Late stage manifestations of ND include corneal opacification and eventual phthisis bulbi.2 The disease progresses to bilateral loss in global volume by the end of the first decade of life.2,22
A majority of patients will lose all light perception during the first year of life.2,5 Less than 20% will have light perception after 3 years of life.5 To date, the oldest person with a pathogenic NDP mutation associated with ND with intact light perception was 87 years at time of testing.13 Of note, this man had never manifested any ocular or extraocular manifestations of ND and was only screened due to early-childhood blindness in his grandson.13
Therapeutic Considerations
Genetic Testing
In affected males, sequence analysis of NDP can detect up to 95% of pathogenic variants.2 If an affected patient has a negative sequence analysis, deletion/duplication analysis should be considered.2
For women who are concerned with their carrier status, screening should only be performed if a ND patient can be identified in their family history. For women, screening should also begin with sequence analysis and deletion/duplication analysis.2
If no pathogenic variant is detected on sequence or deletion/duplication analysis, linkage analysis for markers linked to NDP can be considered.2 However, because linkage analysis often requires many family members over several generations to be tested, it may not be feasible for all families.
In simplex cases, because there is phenotypic overlap with mutations in the Wnt signaling pathway, sequencing of other genes involved in Wnt signaling should be explored. These include TSPAN12, KIF11, CAPN5, LRP5, and FZD4, ZNF408. Several commercial CLIA-certified labs provide an exudative vitreoretinopathy panel, and the latest panel and pricing information may be found at https://www.genetests.org.
Since the field of molecular and genetic testing is ever evolving, families should be asked to consider DNA banking so that their genetic material can be readily assessable as new, more effective testing is created.
Genetic Counseling
Patients with suspected ND should be evaluated by a genetic counselor to identify risks for certain disorders, investigate family health history, and interpret and explain testing results. Genetic counselors usually work in concert with an ophthalmic geneticist and/or human geneticist.
Ocular Surgical Intervention
All pediatric patients with leukocoria should be referred to an ophthalmologist for a full evaluation to rule out retinoblastoma. ND patients should also have regular ophthalmology follow-up, even in the setting of profound vision loss, to monitor for phthisis and possible chronic pain.
Unfortunately, for patients who present at birth with complete retinal detachment, surgery is usually not an option.2 For patients with partial retinal detachments, there is no current standard of care; however, laser photocoagulation and vitrectomy have been proposed as possible early interventions to preserve some vision.21,23
A retrospective study of 11 patients with ND published by Walsh et al in 2010 demonstrated that vitrectomy performed in the first 3-4 months of life preserved at least light perception in one eye in 8 patients.22 They proposed vitrectomy-released traction on the retina and allowed for improved retinal development. They also hypothesized that patients with complete retinal detachments should still be considered for vitrectomy to release retinal traction and prevent the loss of intraocular pressure and development of phthisis bulbi.
In 2010, Chow et al reported the first case of prophylactic laser coagulation in a neonate delivered at 37 weeks with an NDP mutation identified by amniocentesis.21 In their study, they reported no retinal detachment at 24 months of age and a corrected Teller visual acuity of 20/100 bilaterally at 23 months, leading the group to propose that early detection of NDP mutations in pregnancy could allow for early surgical interventions to preserve vision in those with ND disease.21 Salient features in addition to avascular peripheral retina in this patient included prominent vasa hyaloidae propria and macular hypoplasia with atypical vasculature. While this mutation was not in the cysteine knot motif, the phenotype of affected members within this pedigree was consistent with ND.24 In 2014, Sisk and colleagues published the first planned preterm delivery of a patient with a pathogenic NDP mutation identified by amniocentesis with prompt laser photocoagulation and VEGF therapy at 34 weeks (Figure 2).23 Of note, the patient had an affected uncle and older brother, the latter of whom was born with bilateral retinal detachments at 38 weeks.23 Therefore, the most opportune time to treat may be prior to natural birth.23 In both cases of early laser treatment, there was incomplete foveal development with low vision in both eyes.23
For palliative care, surgery may also be required for increased ocular pressure and eye pain.2 Although rare, enucleation may be considered for a blind, painful eye.2
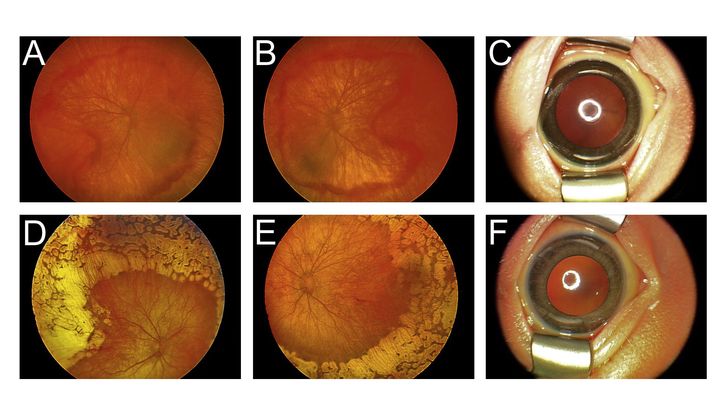
Figure 2. Fundus photograph of 34-week-old infant with Norrie Disease.
A and B. Pretreatment images of the right and left eye demonstrating incomplete retinal vasculogenesis with neovascularization and hemorrhage, and incomplete foveal vascularization. C. The tunica vasculosa lentis was prominent. D and E. Sixteen weeks post-treatment images demonstrate near confluent laser, reduction in vessel caliper, regression of neovascularization and resolution of hemorrhages with the exception of the temporal untreated border. The fovea was spared in the right eye and the macula was spared in the left eye from laser completely. F. Regression of the tunica vasculosa lentis after treatment. Image reproduced with permission from Elselvier, Sisk et al., "Planned Preterm Delivery and Treatment of Retinal Neovascularization in Norrie Disease." Ophthal. 112.6 (2014).
Ocular Non-surgical Intervention
Patients with ND require early intervention with low vision services to ensure accommodations are made to promote learning and development. Vision services should be offered within the first year of life and individualized educational plans (IEPs) implemented in the preschool years.
Sensorineural Hearing Loss Intervention
Since the onset of hearing loss has been linked to depression in ND patients,5 it is important to have early and regular hearing screens so that patients can be connected to resources and support networks expeditiously.
For severe impairment, cochlear implants in adulthood are a viable option.5,18 In a 2012 study, 4 patients with ND underwent successful cochlear implants and reported significantly improved quality of life.5
Cognitive/Behavioral Interventions
Unfortunately, there have been no studies on the efficacy of psychotropic drugs in ND patients.2 However, considering early psychiatric support and school involvement (eg, IEPs) may help relieve patient and family stress and maximize the patient’s potential.
NDP-related retinopathologies
Familial exudative vitreoretinopathy (FEVR)
Mechanism: Missense mutations in NDP alter norrin-frizzled-4 binding, resulting in abnormal blood supply of the retina.25 Mutations in other genes involved in Wnt signaling such as TSPAN12, KIF11, CAPN5, LRP5, FZD4, and ZNF408 have also been identified.25-27
Manifestations: Peripheral avascularity of the retinas that progresses to neovascularization, subretinal fluid accumulation, and eventual retinal detachment.28
Persistent Fetal Vascular Syndrome (PFVS)
Previously: Persistent hyperplastic primary vitreous
Mechanism: Associated gene(s) unknown. Bilateral cases may have a molecular basis including NDP29 and LRP5.30 Hyaloidal stalk remnants attach the retina to the lens, causing tractional detachment of the retina.31
Manifestation: Usually unilateral leukocoria, fibrous stalk in vitreous, and retinal detachment. Bilateral PFV has been associated with ND.2
Retinopathy of prematurity (ROP)
Mechanism: Norrin has been shown to be protective against oxidative damage of retinal cells in mice,20 and thus may be protective against the oxidative damage seen in ROP. Furthermore, NDP mutations increase the risk of retinal detachment due to abnormal blood vessels formation.32-34
Manifestation: Abnormal retinal neovascularization secondary to immature peripheral retinal vasculature that can lead to retinal detachment and long-term visual impairment.35
References
- Norrie Disease. 2007; https://ghr.nlm.nih.gov/condition/norrie-disease. Accessed June, 2017.
- Sims KB. NDP-Related Retinopathies. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews®. Seattle (WA): University of Washington, Seattle. University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved; 1993.
- Drenser KA, Fecko A, Dailey W, Trese MT. A characteristic phenotypic retinal appearance in Norrie disease. Retina. 2007;27(2):243-246.
- Wu WC, Drenser K, Trese M, Capone A, Jr, Dailey W. Retinal phenotype-genotype correlation of pediatric patients expressing mutations in the Norrie disease gene. Arch Ophthalmol. 2007;125(2):225-230.
- Smith SE, Mullen TE, Graham D, Sims KB, Rehm HL. Norrie disease: extraocular clinical manifestations in 56 patients. Am J Med Genet A. 2012;158A(8):1909-1917.
- Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. Mutational analysis of Norrin-Frizzled4 recognition. J Biol Chem. 2007;282(6):4057-4068.
- Ohlmann A, Tamm ER. Norrin: molecular and functional properties of an angiogenic and neuroprotective growth factor. Prog Retin Eye Res. 2012;31(3):243-257.
- Rehm HL, Zhang DS, Brown MC, et al. Vascular defects and sensorineural deafness in a mouse model of Norrie disease. J Neurosci. 2002;22(11):4286-4292.
- Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883-895.
- Ye X, Wang Y, Cahill H, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139(2):285-298.
- Khan AO, Aldahmesh MA, Meyer B. Correlation of ophthalmic examination with carrier status in females potentially harboring a severe Norrie disease gene mutation. Ophthalmology. 2008;115(4):730-733.
- Parzefall T, Lucas T, Ritter M, et al. A novel missense NDP mutation [p.(Cys93Arg)] with a manifesting carrier in an austrian family with Norrie disease. Audiol Neurootol. 2014;19(3):203-209.
- Allen RC, Russell SR, Streb LM, Alsheikheh A, Stone EM. Phenotypic heterogeneity associated with a novel mutation (Gly112Glu) in the Norrie disease protein. Eye (Lond). 2006;20(2):234-241.
- Warburg M. Norie's disease (atrofia bulborum hereditaria). Acta Ophthalmol (Copenh). 1963;41:134-146.
- Halpin C, Owen G, Gutiérrez-Espeleta GA, Sims K, Rehm HL. Audiologic features of Norrie disease. Ann Otol Rhinol Laryngol. 2005;114(7):533-538.
- Ye X, Smallwood P, Nathans J. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expr Patterns. 2011;11(1-2):151-155.
- Okumura A, Arai E, Kitamura Y, et al. Epilepsy phenotypes in siblings with Norrie disease. Brain Dev. 2015;37(10):978-982.
- Norrie Disease 2017; https://rarediseases.info.nih.gov/diseases/7224/norrie-disease. Accessed June, 2017.
- Braunger BM, Tamm ER. The different functions of Norrin. Adv Exp Med Biol. 2012;723:679-683.
- Ohlmann A, Seitz R, Braunger B, Seitz D, Bösl MR, Tamm ER. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci. 2010;30(1):183-193.
- Chow CC, Kiernan DF, Chau FY, et al. Laser photocoagulation at birth prevents blindness in Norrie's disease diagnosed using amniocentesis. Ophthalmology. 2010;117(12):2402-2406.
- Walsh MK, Drenser KA, Capone A, Jr, Trese MT. Early vitrectomy effective for Norrie disease. Arch Ophthalmol. 2010;128(4):456-460.
- Sisk RA, Hufnagel RB, Bandi S, Polzin WJ, Ahmed ZM. Planned preterm delivery and treatment of retinal neovascularization in Norrie disease. Ophthalmology. 2014;121(6):1312-1313.
- Drenser KA, Walsh MK, Capone A, Jr., Trese MT, Luo CK. Preterm treatment of Norrie disease. Ophthalmology. 2011;118(8):1694-1695; author reply 1695,1695 e1691.
- Nikopoulos K, Venselaar H, Collin RW, et al. Overview of the mutation spectrum in familial exudative vitreoretinopathy and Norrie disease with identification of 21 novel variants in FZD4, LRP5, and NDP. Hum Mutat. 2010;31(6):656-666.
- Poulter JA, Davidson AE, Ali M, et al. Recessive mutations in TSPAN12 cause retinal dysplasia and severe familial exudative vitreoretinopathy (FEVR). Invest Ophthalmol Vis Sci. 2012;53(6):2873-2879.
- Yang H, Li S, Xiao X, Wang P, Guo X, Zhang Q. Identification of FZD4 and LRP5 mutations in 11 of 49 families with familial exudative vitreoretinopathy. Mol Vis. 2012;18:2438-2446.
- Sizmaz S, Yonekawa Y, T Trese M. Familial Exudative Vitreoretinopathy. Turk J Ophthalmol. 2015;45(4):164-168.
- Payabvash S, Anderson JS, Nascene DR. Bilateral persistent fetal vasculature due to a mutation in the Norrie disease protein gene. Neuroradiol J. 2015;28(6):623-627.
- Kheir V, Munier FL, Aubry-Rozier B, Schorderet DF. Potential blindness in children of patients with hereditary bone disease. Osteoporos Int. 2016;27(2):841-844.
- NDP Gene. 2009; https://ghr.nlm.nih.gov/gene/NDP. Accessed June, 2017.
- Hiraoka M, Berinstein DM, Trese MT, Shastry BS. Insertion and deletion mutations in the dinucleotide repeat region of the Norrie disease gene in patients with advanced retinopathy of prematurity. J Hum Genet. 2001;46(4):178-181.
- Shastry BS. SNPs: impact on gene function and phenotype. Methods Mol Biol. 2009;578:3-22.
- Shastry BS, Pendergast SD, Hartzer MK, Liu X, Trese MT. Identification of missense mutations in the Norrie disease gene associated with advanced retinopathy of prematurity. Arch Ophthalmol. 1997;115(5):651-655.
- Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2013;368(12):1162-1163.