AUG 21, 2017
By Razek Georges Coussa, MD; Virginia M. Utz, MD; Elias I. Traboulsi, MD
A Compendium of Inherited Disorders and the Eye, Oxford University Press
Genetics
OMIM Numbers
- Stargardt Disease 1 (STGD1) # 248200 [ABCA4]
- Stargardt Disease 3 (STGD3) # 600110 [ELOV4]
- Stargardt Disease 4 (STGD4) # 603786 [PROM1]
Inheritance
Gene
-
Stargardt Disease (STGD) is most commonly caused by mutations in the ABCA4 gene located on chromosome 1 (OMIM 601691) and is inherited in an autosomal recessive manner. The gene encodes for an ATP-binding cassette membrane protein in the retinal rod and cone photoreceptor outer segments involved in the transport of all-trans-retinal aldehyde (OMIM 601691). Mutations in this gene result in accumulation of N-retinylidene-N-retinyl-ethanolamine (A2E), a component of lipofuscin that is known to be toxic to RPE and photoreceptors.1,2 While a majority of cases are caused by autosomal recessive mutations in ABCA4 (STGD1, OMIM 248200), autosomal dominant mutations in ELOVL4 (STGD3, OMIM 605512) on chromosome 6 and PROM1 mutation on chromosome 4 (STGD4, OMIM 604365) can also cause Stargardt disease. In some cases, PROM1 may be inherited in an autosomal recessive manner.3
Epidemiology
- STGD is the most commonly [Mendelian] inherited childhood and adulthood maculopathy.4 It has a prevalence of 1 in 10,000.5 STGD is the second most common cause in the pediatric population, after Leber Congenital Amaurosis in the English working population.6
Clinical Findings
- Patients with STGD typically present in childhood or early adolescence, but some may present in later adulthood. Initial symptoms include bilateral central visual loss characterized by blurred vision, central scotomas, and/or dyschromatopsia. The visual acuity (VA) at presentation varies from 20/20 to 20/400, with earlier age of onset having a worse visual prognosis.7,8 In up to two-thirds of patients, the macula is classically described as “beaten bronze metal” appearance with yellowish, “fish-tail” or pisciform lesions in up to two-thirds of patients (Figure 1).7,8 Interestingly, classic flecks are absent in approximately 30% of patients with childhood onset disease.9 The maculopathy then progresses slowly to a characteristic atrophic macular degeneration classically resulting in a “bull’s eye” pattern (Figure 2).
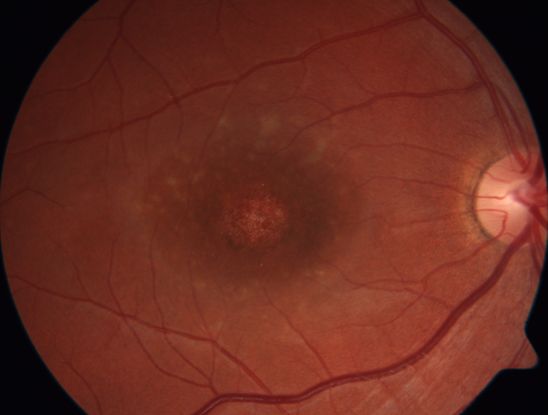
Figure 1. Right macula of a young patient with Stargardt disease showing beaten bronze appearance, a small area of atrophy and some perifoveal flecks.
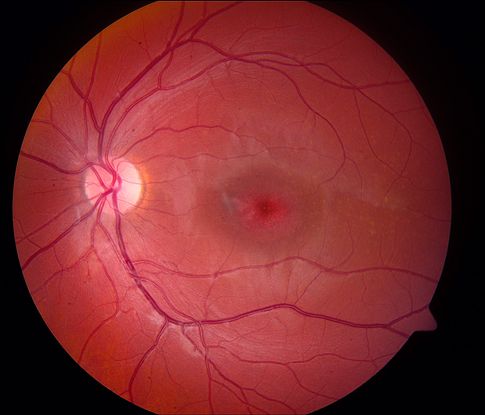
Figure 2. Bull’s eye appearance of the macula in a patient with Stargardt disease
- STGD has been phenotypically characterized into 4 stages by Fishman et al.10 Ophthalmoscopically, stage 1 is essentially characterized by macular pigmentary changes and irregular pigmentary mottling (flecks) located within 1 disc diameter of the fovea.11 About two-thirds of patients presenting at stage 1 remain in this stage over a follow-up period of 5.4 years.12 Stage 2 is identified when these pigmentary changes are identified beyond the vascular arcades temporally and often extending nasally to the optic nerve. Stage 3 occurs when the previously reported flecks resorb and are replaced by atrophic macular changes. Patients presenting in stages 2 and 3 were reported to remain stable over follow-up periods of 7.2 and 5.3 years in about 70% and 86% of cases, respectively.12 Stage 4 is characterized by extensive atrophic changes. This classification is summarized in Table 1.
|
Table 1: Genotypic and Phenotypic Classifications of Stargardt Disease.
|
|
Fishman STGD Classification10
|
Early Onset STGD Fundus Grading System9
|
ERG20
|
FAF16
|
Genotype Group
|
|
Stage 1:
* Pigmentary changes in the macula or pisciform ring of flecks within 1 DD on all sides of the fovea
* ERG and EOG are normal
Stage 2:
* Pisciform flecks present beyond 1 DD of the fovea, often extending beyond the arcades and nasal to the optic disc
* ERG and EOG normal, but cone/rod response may be subnormal
Stage 3:
* Diffusely resorbed flecks and choriocapillaris atrophy of the macula
* EOG subnormal
* ERG subnormal cone or rod amplitudes
* Central field defects + midperipheral/peripheral field defect
Stage 4:
* Diffusely absorbed flecks and choriocapillaris/RPE atrophy throughout the entire fundus
* ERG with reduced rod and cone amplitudes
* Peripheral fields with moderate to extensive restriction
|
Grade 1:
Normal fundus
Grade 2:
Macular and/or peripheral flecks without central atrophy
Grade 3a:
Central atrophy without flecks
Grade 3b:
Central atrophy with macular and/or peripheral flecks
Grade 3c:
Paracentral atrophy with macular and/or peripheral flecks, without central atrophy
Grade 4:
Multiple extensive atrophic changes of the RPE, extending beyond the vascular arcades
|
Group 1:
Pattern ERG (PERG) abnormality with normal full-field ERG (ffERG)
Group 2:
PERG abnormality with cone ERG abnormality
Group 3:
PERG abnormality with generalized cone and rod dysfunction
|
Type 1:
Localized low FAF signal at the fovea surrounded by a homogeneous background with or without perifoveal foci of high or low signal
Type 2:
Localized low FAF signal at the macula surrounded by a heterogeneous background and widespread foci of high or low FAF signal extending anterior to the vascular arcades
Type 3:
Multiple areas of low FAF signal at posterior pole with heterogeneous background and/or foci of high or low signal
|
Genotype A:
Two or more deleterious variants
Genotype B:
One deleterious variant and > 1 missense or inframe insertion or deletion
Genotype C:
Two or more missense or in-frame insertion or deletion variants
|
- VA in STGD is highly correlated with both the diagnostic stage and the patient age at the initial diagnosis. In general, stage 1 STGD patients have VA better than 20/200, while those in stages 2, 3, and 4 have a VA of 20/200 of worse.12 In particular, patients initially diagnosed before the age of 20 years have an average VA decline from 20/ 40 to 20/200 over a follow-up period of 7 years.7 In contrast, VA decline is less progressive in those presenting between ages 21-40 and 41-60 years, whose VA declines over approximately 22 and 29 years, respectively.7 Not surprisingly, patients with macular sparing have a VA of 20/40 or better.7
Imaging and Ancillary Studies
-
In addition to clinical evaluation, fluorescein angiography (FA), fundus autofluorescence (FAF), spectral domain optical coherence tomography (SD-OCT), and electroretinography (ERG) are utilized to diagnose and to characterize the clinical phenotype (Table 1). Some of the imaging findings may have prognostic implications as well.
-
Fluorescein Angiography:
Previously, fluorescein angiography (FA) was considered the “gold standard” diagnostic modality to diagnose STGD based on the “dark choroid” sign present in 60% of patients (Figure 3). The “dark” or “silent choroid” sign results from blockage of early choroidal fluorescence by lipofuscin deposition within the RPE.13

Figure 3. Fluorescein angiography of a patient with Stargardt disease demonstrating the “dark choroid” sign caused by blockage of normal choroidal flush by lipofuscin deposition within the RPE. There is a ring of hyperfluorescence in the perifoveal region corresponding to loss of the RPE in that location.
-
Fundus Autofluorescence (FAF):
Currently, FAF has gained popularity over FA, as it allows early identification of RPE pathology prior to clinical manifestations.14 Both areas of hypo- and hyperautofluorescence are typical of STGD. Specifically, there is initially a hyperautofluorescent signal, which is likely due to lipofuscin accumulation in the RPE, progressing over the disease course to hypoautofluorescence, which is thought to result from RPE death.15 Fujinami et al. classified FAF patterns into 3 subtypes based on the degree of low foveal FAF signal and the extent of high or low FAF extending perifovea and/or the arcades (Table 1 and Figure 4).16 From this classification system, the authors measured the areas of atrophy and calculated the rate of atrophy enlargement [RAE] per year.16 Subtype 1 has the slowest rate of progression and is more likely to be associated with milder sequence variations (eg, missense), whereas types 2 and 3 are likely to progress more rapidly and are associated with more deleterious mutations (eg, nonsense mutations).16 Thus, baseline FAF has important prognostic implications for counseling patients and has become increasingly important to monitor patients enrolled in clinical treatment trials.
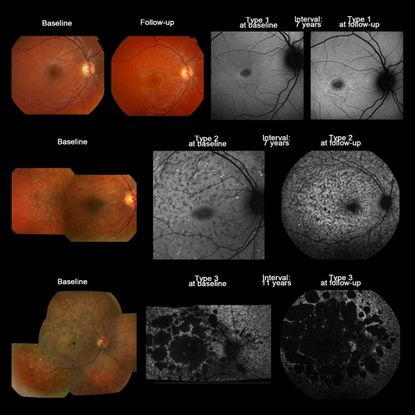
Figure 4. Color fundus photographs and AF images of 3 representative cases with Stargardt disease, illustrating the 3 AF subtypes in subjects where there was no subtype transition over time (patients 7, 50, and 54). Top row: Color fundus photographs of patient 7 showing subtle RPE changes at the fovea at baseline with a mild increase in the degree of atrophy at follow-up. AF imaging demonstrates a localized low signal lesion with a relatively high signal edge and a homogeneous background at baseline, and a low signal foveal lesion surrounded by patchy small foci with high signal and a homogeneous background at follow-up; consistent with AF type 1 at baseline and follow-up. Middle row: Patient 50 had macular atrophy surrounded by yellowish-white flecks extending anterior to the vascular arcades at baseline, and a low signal area at the macula surrounded by high and low foci throughout the posterior pole (AF type 2) at baseline and follow-up, with a heterogeneous background at baseline and follow-up. Bottom row: Patient 65 had extensive areas of atrophy throughout the posterior pole, extending beyond the vascular arcades, with yellowish-white and atrophic flecks at baseline, and multiple areas of low signal with heterogeneous background (AF type 3) at baseline and follow-up. (Reproduced with permission from Figure 1. Fujinami K, et al., Invest Ophthalmol Vis Sci. 2013;54:8181-819016)
-
Spectral-Domain Optical Coherence Tomography (SD-OCT):
Optical coherence tomography (OCT) provides cross-sectional images of the macula. The hallmark of STGD is outer retinal foveal atrophy and loss of the inner ellipsoid band (Figure 5). Parameters such as total retinal thickness, macular volume, outer retinal thickness, and inner segment ellipsoid loss can be monitored longitudinally over time.17 The earliest OCT finding in children is thickening of the external limiting membrane prior to outer retinal atrophy.18,19
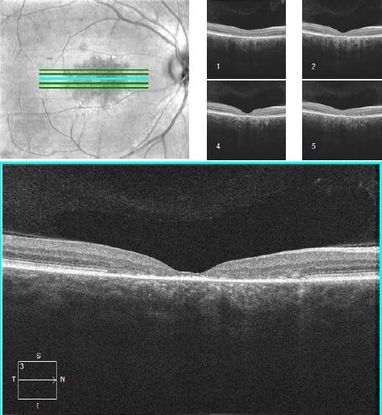
Figure 5. OCT demonstrating outer retinal foveal atrophy and loss of the inner segment ellipsoid in the fovea of a patient with Stargardt disease.
-
Electroretinography:
The electroretinogram (ERG) in STGD is variable and generally can be classified in 3 distinct groups (Table 1). Group 1 has a normal scotopic and photopic ERG with abnormal pattern ERG (PERG).20 Group 2 is characterized by photopic ERG abnormalities, while group 3 has both photopic and scotopic ERG abnormalities.20 Importantly, full-field ERG (ffERG) findings at baseline have prognostic implications. Those with initial evidence of rod dysfunction were more likely to have progressive ERG deterioration over an average of 10 years of follow-up than those with a normal ERG at baseline.21
-
Adaptive Optics Scanning Light Ophthalmoscopy:
More recently, adaptive optics scanning light ophthalmoscopy (AOSLO) is an emerging research imaging modality that allows visualization of the retina at the cellular level. Two types of AOSLO imaging exist, confocal and split-detector (SD, non-confocal). Confocal imaging requires an intact photoreceptor outer segment, and dark spaces represent cellular vs. outer segment loss. To further differentiate, SD-AOSLO requires only the presence of intact inner segments.22 In STGD, dark spaces on confocal AOSLO may demonstrate intact inner segments on SD-AOSLO, albeit "swollen" on split-detector imaging.23 Thus, the status of the outer and inner segments may have implications for timing of and candidacy for therapeutic interventions.
Genotype-Phenotype Correlates
-
Currently, there are more than 900 disease-causing mutations with differing, but overlapping clinical manifestations.24 This makes STGD a highly phenotypically heterogeneous disease. Variations in ABCA4 can be associated with cone, cone-rod, or rod-cone phenotype.25 Even among families with the same mutations, phenotypes may be discordant, implicating other genetic or environmental factors in the pathophysiology of disease.26,27 In general, those with missense variations tend to have milder, later onset disease, whereas those with null mutations (nonsense) tend to have more severe early onset disease. Homozygosity for missense mutations, Gly1961Glu or Leu2027Phe are associated with milder, older onset disease.28 However, specific missense mutations may be associated with severe disease much like null mutations (eg, complex allele pLeu541Pro and p.Ala1038Val and p.Arg1640Trp).28 The mildest form of STGD is the foveal-sparing subtype (FS-STGD1) is associated with hypomorphic alleles such as p.Gly863Ala29 and p.Asn1868Ile29 and p.Arg2030Gln.30 Importantly, studies have demonstrated that those with > 2 ABCA4 variants identified may have early onset and more severe disease.(Fujinami, 2015 #38;Lambertus, 2015 #12).8 This information is very important for patients counselling.
-
STGD is generally not known to be associated with other ocular or systemic finding. There is, however, one exceptional report of STGD presenting in association with mental retardation, corpus callosum hypoplasia, or agenesis, as well as craniofacial dysmorphic features, including prominent ear lobules, flared eyebrows, and high-arched palate.31
Therapeutic Considerations
- Currently, there is still no proven treatment to halt or reverse STGD-related maculopathy. Early diagnosis is essential for low vision interventions, vocational and educational modifications, and family planning. In some cases, the genotype may have prognostic significance. Nonetheless, several promising treatment options are being investigated in humans. Essentially, the treatment options targeting STGD maculopathy are gene replacement therapies, stem cells therapies, or pharmacologic therapies.
- Gene replacement therapy is a logical means to halt and, possibly reverse, STGD. This therapy is based on introducing a subretinal injection of adeno-associated virus (AAV) vectors genetically engineered with ABCA4-DNA. Presently, there are 2 clinical trials investigating the use of StarGenTM, which is an equine infectious anemia virus lentiviral vector, in order to reduce A2E accumulation in STGD.32 The first is phase I/II clinical trial (ClinicalTrials.gov Identifier: NCT01367444) that has demonstrated safety; however, evidence of efficacy is limited in patients with severe disease.33,34 The study is currently recruiting patients with less severe disease to determine efficacy and best timing for intervention. The second trial is another ongoing safety and tolerance phase I/II clinical trial (ClinicalTrials.gov Identifier: NCT01736592) projected over 20 years.
- Human embryonic stem cells (hESCs) is another promising therapeutically modality aimed to regenerate dysfunctional RPE cells. Injection of hESCs into mouse models results in successful RPE integration.35 Currently, 2 safety and tolerance phase I/II clinical trials are being conducted to assess the safety and tolerance of hESC-RPE cells subretinally transplanted in advanced STGD (ClinicalTrials.gov Identifiers: NCT01345006/NCT01469832, Astellas Institute for Regenerative Medicine).36
- Oral substitutes of key components of the visual pathway are also viable therapeutic options for STGD maculopathy. In particular, oral administration of ALK-001, which is a deuterated vitamin A (C20-D3-vitamin A, Alkeus Pharmaceuticals), has been shown to reduce the accumulation of cytotoxic A2E and lipofuscin by chemically slowing the vitamin A dimerization rate in mice model.37 Currently, ALK-001 is subjected to a phase II clinical trial (ClinicalTrials.gov Identifier NCT02402660) in STGD patients. Another potential oral supplement is fenretinide, which is a synthetic retinoid-based RBP4 antagonist (N-(4-hydroxyphenyl) retinamide, 4-HPR, Sirion Therapeutics), capable of reducing vitamin A retinal concentrations and thus reducing the A2E formation. Preclinical STGD mouse experimentation showed dose-dependent promising results.38
References
- Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nature Genet. 15: 236-246, 1997. Note: Erratum: Nature Genet. 17: 122 only, 1997.
- Sparrow JR, Gregory-Roberts E, Yamamoto K, et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31:121–35.
- Permanyer J, Navarro R, Friedman J, et al. Autosomal recessive retinitis pigmentosa with early macular affectation caused by premature truncation in PROM1. Invest Ophthalmol Vis Sci. 2010;51(5):2656-2663.
- Michaelides M, Hunt DM, Moore AT. The genetics of inherited macular dystrophies. J Med Genet. 2003;40:641–650.
- Blacharski PA. Fundus flavimaculatus. In: Newsome, DA. (ed.): Retinal Dystrophies and Degenerations. New York: Raven Press 1988. 135-159.
- Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014;4:e004015.
- Rotenstreich Y, Fishman GA, Anderson RJ. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology 2003; 110(6):1151–1158.
- Miraldi Utz V, Coussa RG, Marino MJ, et al. Predictors of visual acuity and genotype-phenotype correlates in a cohort of patients with Stargardt disease. Br J Ophthalmol. 2014;98(4):513-518.
- Fujinami K, Zernant J, Chana RK, et al. Clinical and molecular characteristics of childhood-onset Stargardt disease. Ophthalmology. 2015;122(2):326-334.
- Fishman GA. Fundus flavimaculatus. A clinical classification. Arch Ophthalmol. 1976;94(12):2061-2067.
- Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30(2):63-68.
- Kim LS, Fishman GA. Comparison of visual acuity loss in patients with different stages of Stargardt's disease. Ophthalmology 2006; 113:1748–1751.
- Lambertus S, van Huet RA, Bax NM, et al. Early-onset stargardt disease: phenotypic and genotypic characteristics. Ophthalmology 2015;122:335–44.
- Wabbels B, Demmler A, Paunescu K, et al. Fundus autofluorescence in children and teenagers with hereditary retinal diseases. Graefes Arch Clin Exp Ophthalmol 2006;244:36–45.
- Chen Y. Ratnam K, Sundquist S, et al. Cone photoreceptor abnormalities correlate with vision loss in patients with Stargardt disease. Invest Ophthal Vis Sci. 2011;52: 3281-3292.
- Fujinami K, Lois N, Mukherjee R, et al. A longitudinal study of Stargardt disease: quantitative assessment of fundus autofluorescence, progression, and genotype correlations. Invest Ophthalmol Vis Sci. 2013;54:8181-8190.
- Strauss RW, Muñoz B, Wolfson Y, et al. Assessment of estimated retinal atrophy progression in Stargardt macular dystrophy using spectral-domain optical coherence tomography. Br J Ophthalmol. 2016;100(7):956-962.
- Lee W, Nõupuu K, Oll M, et al. The external limiting membrane in early-onset Stargardt disease. Invest Ophthalmol Vis Sci. 2014;55(10):6139-6149.
- Burke TR, Yzer S, Zernant J, Smith RT, Tsang SH, Allikmets R. Abnormality in the external limiting membrane in early Stargardt disease. Ophthalmic Genet. 2013;34(1-2):75-77.
- Lois N, Holder GE, Bunce C, Fitzke FW, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch Ophthalmol. 2001;119(3):359-369.
- Fujinami K, Lois N, Davidson AE, et al. A longitudinal study of stargardt disease: clinical and electrophysiologic assessment, progression, and genotype correlations. Am J Ophthalmol. 2013;155(6):1075-1088 e1013.
- Scoles D, Sulai YN, Langlo CS, et al. In vivo imaging of human cone photoreceptor inner segments. Invest Ophthalmol Vis Sci. 2014;55(7):4244-4251.
- Song H, Rossi EA, Latchney L, et al. Cone and rod loss in Stargardt disease revealed by adaptive optics scanning light ophthalmoscopy. JAMA Ophthalmol. 2015;133(10):1198-1203.
- Zernant J, Xie YA, Ayuso C, et al. Analysis of the ABCA4 genomic locus in Stargardt disease. Hum Mol Genet. 2014;23:6797–806.
- Klevering BJ, Deutman AF, Maugeri A, Cremers FP, Hoyng CB. The spectrum of retinal phenotypes caused by mutations in the ABCA4 gene. Graefes Arch Clin Exp Ophthalmol. 2005;243(2):90-100.
- Michaelides M, Chen LL, Brantley MA, Jr., et al. ABCA4 mutations and discordant ABCA4 alleles in patients and siblings with bull's-eye maculopathy. Br J Ophthalmol. 2007;91(12):1650-1655.
- Burke TR, Tsang SH, Zernant J, Smith RT, Allikmets R. Familial discordance in Stargardt disease. Mol Vis. 2012;18:227-233.
- Fujinami K, Sergouniotis PI, Davidson AE, et al. The clinical effect of homozygous ABCA4 alleles in 18 patients. Ophthalmology. 2013;120(11):2324-2331.
- Zernant J, Lee W, Collison FT, et al. Frequent hypomorphic alleles account for a significant fraction of ABCA4 disease and distinguish it from age-related macular degeneration. J Med Genet. 2017;54(6):404-412.
- Fujinami K, Sergouniotis PI, Davidson AE, et al. Clinical and molecular analysis of Stargardt disease with preserved foveal structure and function. Am J Ophthalmol. 2013;156(3):487-501 e481.
- Descartes M, Royal SA, Franklin J, Goodin K, Mancuso M, Mikhail FM, Holt L. A new syndrome with Stargardt macular degeneration, abnormalities of the corpus callosum, mental retardation, and dysmorphic features: a case report of two siblings. Clin Dysmorph. 2009;18: 178-180.
- Kong J, Kim SR, Binley K, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–1320.
- Smith J, Ward D, Michaelides M, Moore AT, Simpson S. New and emerging technologies for the treatment of inherited retinal diseases: a horizon scanning review. Eye (Lond) 2015;29:1131–1140.
- Oxford BioMedica (2012). Press release: Oxford BioMedica announces positive DSMB Review of ongoing RetinoStat® and StarGen™ clinical studies. http://www.oxfordbiomedica.co.uk/press-releases/oxford-biomedica-announces-positive-dsmb-review-of-ongoing-retinostat-r-and-stargen-clinical-studies/. Accessed July 12, 2017.
- Lu B, Malcuit C, Wang S, Girman S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27(9):2126-2135.
- Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516.
- Charbel Issa P, Barnard AR, Herrmann P, Washington I, MacLaren RE. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc Natl Acad Sci USA. 2015;112:8415–8420.
- Radu RA, Han Y, Bui TV, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393-4401.