Introduction
Definition
Amblyopia is clinically defined as reduction of visual acuity in one or both eyes, caused by abnormal binocular interaction during the critical period of visual development, that cannot be attributed to any ocular or visual system abnormality or to refractive error.1 The American Academy of Ophthalmology considers amblyopia an interocular difference of 2 lines or more in a visual acuity table (without specifying any), or visual acuity worse than or equal to 20/30 with the best optical correction.2
With an incidence of 3% to 6%, amblyopia is the most common cause of low visual acuity in children and adults in developed countries and has great economic and social impact.3-5 Individuals with amblyopia often have restricted career options and reduced quality of life, including less social contact, cosmetic distress (if associated with strabismus), low self-esteem, visual disorientation, and fear of losing vision in the other eye.5-8
The adoption of interocular difference of visual acuity as a definition contemplates many of the points that concern the different definitions for amblyopia, such as reduction of visual acuity, functional imbalance between the eyes, and inadequate binocular information input in primary visual cortex.9-12
Pathophysiology
Though well-known since antiquity, many neural, physiological, and psychological aspects of amblyopia are still not fully understood.1,13
Classically defined as a decrease in visual acuity (which is clinically easier to assess), reduced contrast sensitivity of high spatial frequencies, and a binocular vision deficit, amblyopia also affects the development of a broad range of neural, sensory, oculomotor, and perceptual functions of vision.11,14,15 Different visual functions are not fully developed at birth; their full development depends on 3 fundamental conditions during the critical period of visual development in infancy: adequate stimuli received from each eye, ocular parallelism (corresponding images), and integrity of the visual pathways.
Disturbances on input of stimuli received by visual cortex during this plastic and unstable stage of visual development prevent proper use of inputs from the involved eye, culminating in amblyopia. The impact on the visual system is closely related to the time at which the visual disturbance begins, and its intensity, type, and duration.
When the visual stimulus disorder is precocious, severe, unidentified, and not reversed in the first months or years of life, it can lead to profound structural modification of the visual neuronal circuit, causing definitive morphological changes in cortical structures of the lateral geniculate nucleus (LGN) and visual cortex, which lead to definitive alterations in the final visual function of amblyopic eyes.16
When the visual stimulus disorder comes later and with less intensity, the normal anatomy construction of the system is maintained, but it is still possible to have active inhibition from neurons of the normal eye on neurons of the affected eye, also leading to functional amblyopia. This neurological mechanism inhibits the image of the affected eye in an attempt to not disturb the processing of the normal eye.17
The pioneering works by Hubel and Wiesel of cortical structures of cats that had one eye sutured at different times during visual development clearly show a difference in anatomy and functioning of cortical visual neurons corresponding to each eye.18-20 The final behavior of visual function in early-onset and late-onset amblyopia is also significantly different.16,17,21,22
Since amblyopia is a visual development disorder, early diagnosis of ocular changes associated with amblyopia is crucial for good visual prognosis because it allows treatment to begin at a stage where the visual neurological pathways are still amenable to stimulation, recovery, and reversal of cortical damage.
The main ocular alterations that predispose to amblyopia are deprivation of visual stimuli (pupil occlusion by ptosis, opacities of optical media, nystagmus, and many others), alteration of sharpness of visual stimuli by refractive changes (high ametropia and/or anisometropia), and non-corresponding images received by each eye (strabismus).
Types of Amblyopia
Deprivation amblyopia
Deprivation happens when eye diseases prevent the light stimulus from reaching the retina, thus forestalling the normal visual process. It also may occur due to anatomic deficits of the retina or optic nerve, or abnormal movement disorders of the eye (nystagmus). When it occurs during the critical period of visual development, it can cause amblyopia. The main diseases that cause this are congenital cataract, blepharoptosis, nystagmus disorders, optic nerve coloboma and hypoplasia, retinal disorders, persistent fetal vasculature; other disease processes can also result in amblyopia.
Amblyopia caused by deprivation was the first type studied in works of Hubel and Wiesel in the 1950s. The authors demonstrated that suturing the eyelids of cats deprived their eyes from receiving visual stimuli, which led to innumerable anatomical and functional changes in the cortical visual pathways. The authors found that these changes were more drastic the earlier, the more intense, and the more prolonged the deprivation.18,23-27
Studies with cats18 and monkeys10,25-27 have shown that the primary alteration of monocular visual deprivation is a change to the cortical ocular dominance columns. In cats, during the most sensitive phase of the critical period, a day of deprivation leads to a slight reduction in vision. Two to 3 days lead to a proportionally much more severe visual reduction, whereas deprivations of more than 6 to 10 days lead to full shift of the cells from the ocular dominance column to the side of the opposing eye, with severe reduction of vision.19,28
In addition to changes in V1, amblyopia is associated with morphological changes in CGL.25,27,29-32
The effect of late eye closure has also been studied. Thus, the effect of deprivation on the size of the bands of the cortical ocular dominance columns was greatly reduced when closure occurred after 10 weeks of age.19,32
Therefore, 3 months would be the end of the critical period of cortical changes in a monkey, which would correspond to approximately 18 months of life in a human.33 At this age, the critical development period has not ended but is susceptible to different changes in the visual system.32 Despite the importance of data from animal models, several authors caution that comparisons between these models and human models should be carefully analyzed. Not only is brain structure different among species, but deprivation studied in animal models is known and controlled, whereas in most cases involving children, there will be varied clinical pictures and multiple associated factors to amblyopia.34
Having taken this into account, many authors proved that deprivation causes different impacts on children’s vision, and that the period and severity of deprivation will bring different deficits to the final visual function.35,36
The ideal period to treat the causes of deprivation in humans is within the first six months of life; after that, the chance to ensure the effectiveness of treatment and achieve normal results decreases rapidly.37 The severity of deprivation makes a difference in these first 6 months. For instance, dense bilateral cataracts not treated by 3 months of age will almost assuredly lead to the development of nystagmus, which will severely limit visual acuity permanently.38
Deprivation amblyopia causes profound anatomical changes in visual circuitry and has the greatest impact on visual acuity and all other visual functions. Its treatment is challenging, and results are generally less successful than in other forms of amblyopia.4,37,39
Anisometropic amblyopia
Anisometropia is a difference in the state of refraction of at least 1 diopter between 2 eyes.40 The prevalence of anisometropic amblyopia is about 4.7% in children and may be myopic, astigmatic, or hypermetropic. The most common type of anisometropia seems to vary with the age, ethnicity, and ocular pathologies of the analyzed sample.41-44
Hypermetropic anisometropia is the most likely type to cause amblyopia, since the retina of the more ametropic eye never receives a clear and defined image: The fovea of the good eye is focused and there will be no stimulus of accommodative effort to adjust the focus of the more hyperopic eye. In myopic anisometropia, the more ametropic eye can be used for near vision, preventing the same levels of amblyopia as seen with hyperopia.1,45,46
Anisometropia may be considered a moderate form of deprivation of visual stimulus, since the more ametropic eye is deprived of receiving a good-quality stimulus in retina. Anatomical and functional changes similar to deprivation are therefore expected in amblyopia caused by anisometropia.47,48
In cases of anisometropia as well as in deprivation, there is a partial "disconnection" of the affected eye in the primary visual cortex, leading to abnormal neuronal competition. While in normal animals most cortical neurons respond to stimulation of 2 eyes, in animals that were subjected to occlusion or blurring of the image of one eye, the proportion of cortical neurons responding to stimuli of the affected eye is much smaller. There is also clear evidence of neural acuity deficit in anisometropia and deprivation. That is, cortical neurons that still respond to stimuli of the affected eye tend to have diffuse and insensitive receptive fields and, therefore, generate worse spatial resolution and contrast sensitivity.30,49,50
The severity of amblyopia is not directly related to the magnitude of the refractive degree itself but to the amount of anisometropia between the 2 eyes. Levi and colleagues demonstrated that VA falls rapidly with increasing magnitude of anisometropia, but that only occurs in high isoametropias with very high refractive levels (> 15D), suggesting that mechanisms other than optical blur, especially abnormal binocular interactions, are involved in the risk of amblyopia.48,51
Despite differences in the inputs received from each eye, in anisometropia both eyes receive congruent images; that is, unlike strabismus there is no stimulation of non-corresponding retinal areas. The suppression is mainly foveal, but the periphery continues to fuse images.52,53 Therefore, pure anisometropic amblyopia classically leads to significant visual acuity deficits compatible with loss of contrast sensitivity of all spatial frequencies, but with relative sparing of binocular vision.11,54,55
Anisometropic amblyopia is often associated with microtropia, leading to a mixed mechanism of visual disturbance.1
Amblyopia by pure anisometropia is the one with the best prognosis, with sometimes surprising recovery of VA with the use of adequate correction alone, and even in later treatments.56 Studies have shown that the presence of preserved or subnormal binocular function is an important factor for the recovery of the system, although the same research has shown that in addition to classic monocular occlusive treatment, other forms of balanced binocular (dichoptic) treatment are ideal for restoring normal visual function.57-59
Strabismic amblyopia
Strabismus is a deviation of one eye with loss of eye parallelism. As a result, the eyes do not receive equal images, leading the visual system to adapt to this change.1
When the visual system is completely formed (when the person reaches adulthood), the perception of non-corresponding images by 2 eyes leads to double vision, but when the visual system is in its critical period of development (in childhood), the brain is still capable of using mechanisms to avoid diplopia or rivalry by inhibiting the activation of the retinocortical pathways originating from the fovea of the deviating eye. This adaptive mechanism avoids diplopia, but it causes a restructuring of the visual cortical circuits in the visual cortex that in turn causes amblyopia.
In strabismic amblyopia, the cortical ocular dominance columns remain structured, even in cases of moderate amblyopia. Only in cases of deep amblyopia are there reports of alteration of dominance columns.25,26,60-65
Although the cortical cellular apparatus is relatively preserved, many functional changes occur in the visual system. There is active and deep suppression of the dominant eye over the deviating eye, retinal correspondence is completely lost, and cellular interactions are altered.
Tychesen and colleagues have shown many visual function alterations in monkeys with strabismus, as well as loss of V1 binocular connections.25-27,65 The severity of motor ocular changes and the loss of V1 binocular connections increased as a function of the duration of decorrelation. The animals exposed to only 3 weeks of decorrelation recovered these functions. Other studies have shown that excitatory interactions for the deviating eye remain deactivated, but inhibitory ones do not, even after correction of the position of the deviating eye, indicating active cortical suppression and an imbalance between the cortical cellular columns.66-68
Strabismus causes change in or loss of connectivity to the cortical spatial information pathways, altering the spatial summation and side inhibitions of received stimuli and, consequently, preventing the integration of contours and shapes. A distortion of the spatial vision occurs that interferes with numerous discriminatory visual tasks including visual acuity, Vernier visual acuity (alignment accuracy), and crowding.69-74
In strabismus there is no binocular facilitation for any type of stimulus; the suppression is constant and strong and is probably a modified form of suppression of binocular rivalry.53 Suppression is also found in the fovea of the normal eye when the amblyopic eye is fixing, showing that lost visual acuity is not related solely to suppression. Thus, it is suppression that leads to amblyopia in an individual who has strabismus and not vice versa, because the inactivity of the system may interfere with the process of synaptic development.67
In strabismus, the different stimuli received by the eyes prevent normal image fusion, compromising binocular vision and summation and the ability to discriminate disparity and depth of vision with altered stereoscopic visual acuity (stereopsis) and even postural stability.6,60,75-81
Contrast sensitivity in strabismic amblyopia is less affected than in amblyopia due to deprivation or anisometropia, with change mainly to high spatial frequencies.82
Amblyopia caused by strabismus therefore has a major impact on visual acuity and binocular vision, and contrast sensitivity is relatively sparing.11
Mixed amblyopia
Amblyopia is considered mixed when caused by 2 amblyogenic factors. Combination of anisometropic and strabismic amblyopia is common, especially in partially accommodative esotropia, microtropia, and monofixation syndrome.1,55
Clinically, mixed amblyopia is more severe with similar deficits of visual functions, there is an exacerbation of visual acuity loss and contrast sensitivity and typically an extinction of stereopsia. The magnitude of the impact on each visual function will depend on the concomitant onset or at different times of each ocular change.6
A study comparing visual acuity, Vernier acuity, grating acuity, contrast sensitivity, and binocular function of adults with amblyopia of different etiologies (11 categories) with normal subjects revealed 2 main dimensions of variation of visual performance on subjects with amblyopia: one related to visual acuity measures (optotypes, Vernier, and grids) and the other related to contrast sensitivity measurements (Pelli-Robson and edge contrast sensitivity).47 The authors have demonstrated different distributions of visual loss for different amblyopia categories and have suggested that 2 consequences of associated conditions—resolution reduction and loss of binocularity—determine the visual deficit pattern.47
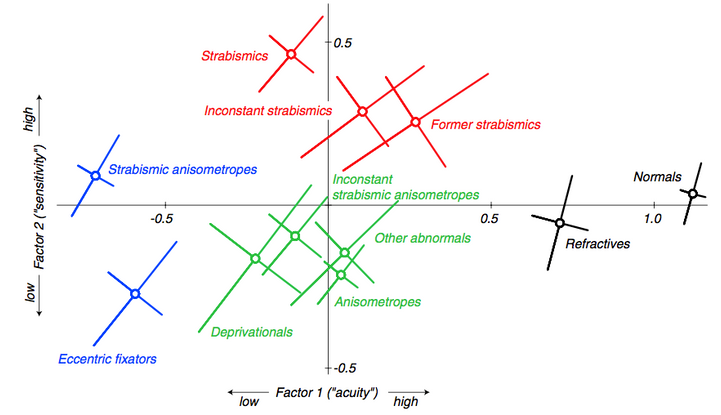
Figure 1. Amblyopia Map: The figure shows the mean locations of the 11 clinically defined subject categories in the two-factor space. The diagonal bars show one standard error of the mean measured along the principal axes of the elliptical distributions. (Reprinted with permission from McKee et al. [2003]).
Other Cortical Areas and Complex Functions Affected by Amblyopia
Amblyopia is, therefore, a neural disorder resulting from abnormal brain stimulation during the critical period of visual development. As shown by several studies, the striate cortex (V1) is the main cortical area affected by amblyopia. Amblyopes have decreased binocular neurons and decreased neurons responsible for the amblyopic eye in V1 in addition to active binocular suppression.23,30,61,65,83-87
Despite the well-known visual processing deficits, recent work has shown that amblyopic patients present alterations in visual processing of high-order cortical functions88 such as deficiency in movement integration,89 perception and processing of shape and global contour,13,90-92 altered perception of alignment (Vernier acuity), and symmetry.93,94 Deficits in tasks involving high-order attention components have also been described72,95-102 as enumeration of objects, prolonged attentional blinking, the "crowding" phenomenon, the reading process, and visual decision-making. Recent evidence shows that the perceptual impact of amblyopia extends even beyond vision to multisensory processing.103 Abnormalities are evident in audiovisual speech perception,104-106 spatial audiovisual localization,107 and temporal judgment tasks.108
These high-order deficits are also found in the fellow eye72,109-113 and during binocular vision.98,103,114,115
The element common to all of these affected sensory-motor tasks is they are not limited to acuity, and that they require both local and global cortical processing91,116 and involve extraction and segregation of a background noise signal,117-119 clearly implicating high-order visual processes.
To confirm these deficits found by electrophysiological and psychophysical research, new technologies such as positron emission tomography,120,121 magnetoencephalography,122 anatomic and functional magnetic resonance imaging (MRI), and functional magnetic resonance imaging (fMRI)54,123-128 are being used to investigate the sites and extent of cortical deficits in humans with amblyopia.103
These studies show alterations of activation in both V1 and extra-striate areas of the visual pathways of the amblyopic patient (ventral: V2, V4, V8, and dorsal: mean temporal area [MT] / V5), and show that activity transmission of the amblyopic eye is progressively affected the higher the processing level.54,124,126,129
Studies with fMRI are also confirming different impacts on visual cortex related to different types of amblyopia. Recent findings suggest a more profound disorganization of the cortical arrangement in patients with strabismic amblyopia, in which the interhemispheric asymmetry for parvo- and magnocellular input processing was lost, whereas normal cortical asymmetry was present in those with anisometropic amblyopia.130-132
Although these studies focus on the location of visual changes, it should be noted that cortical activity depends on constant interaction between different brain areas, and it is imperative to determine if high-processing areas inherit abnormalities from lower levels of processing or if there are developmental abnormalities in extra-striate that may have direct consequences on visual processing.89 Therefore, a better understanding of amblyopia requires investigation of how amblyopia is associated with abnormal interactions between various brain areas and how these feedback and feedforward interactions are affected.6,114,133
Recent research has shown that amblyopia leads to abnormality of multisensory brain processing that persists even in a binocular condition. Richards and colleagues demonstrated in their experiments alterations in the temporal, spatial, and speech audiovisual perception in amblyopic subjects, indicating that amblyopia causes multisensorial brain alteration and not only a uni-sensorial visual impairment.103,107,108
Diagnosis
Despite the varied deficits in visual function, the diagnosis of amblyopia is still done by measuring visual acuity on an eye chart, using optotype-based recognition.
In preverbal children who cannot complete this task, the diagnosis can be made using behavioral methods such as the fixation preference by observing the vigor with which the child objects to occlusion of one eye relative to the other. Grading schemes can be used to quantitatively measure fixation preference,134 and grating acuity can be determined using the Teller acuity cards.135 Recognition visual acuity testing based on optotypes (letters, numbers, or symbols) must be done as soon as the child can perform this task reliably.136
As amblyopia is a common and preventable visual deficit, there is a great concern regarding early diagnosis and in determining more effective treatments for the condition. The American Academy of Pediatrics recommends screening for amblyopia as part of the regular well-child visit made by a pediatrician or family care practitioner, including the use of instrument-based vision screening techniques for preverbal children.137
Randomized longitudinal studies have shown that screening improves vision outcomes, decreasing the prevalence of amblyopia by as much as 60%.138 Moreover, novel technologies, such as instrument-based devices (vision screeners) help primary care providers diagnose amblyopia in the early stages and refer children for specialized ophthalmologic care.139,140 Earlier detection can allow for earlier treatment and will result in better outcomes for those children whose amblyopia is detected early.141
Treatment
The gold standard treatment for amblyopia is patching the better eye to force the brain to use the weaker eye. Depriving the fellow/fixating eye of vision forces the amblyopic eye to strike suppression and to use the visual cortex corresponding to the eye to recover connections for better vision. Alternatives to patching are optical penalization with atropine eye drops, filters to blur the better eye, optical defocus using glasses or contact lenses, and dichoptic video games.
In the last 20 years, PEDIG (Pediatric Eye Disease Investigator Group),142-143 as well as MOTAS (Monitored Occlusion Treatment of Amblyopia Study),144 have conducted randomized clinical trials to address the main issues of occlusive treatment and to define optimal treatment protocols.
The PEDIG studies have published 17 Amblyopia Treatment Studies (ATS) that are evaluating amblyopic treatment for children 3 to 17 years old, and the major results to date are:
- Optical correction alone is successful in improving the amblyopia in nearly 1/3 of patients (anisometropic, strabismic, or mixed)56,145
- Patching is an effective treatment for amblyopia.146
- The ideal number of hours of patching was evaluated. Children 3 to 7 years old with moderate amblyopia were randomized to 2 hours of patching per day compared with 6 hours of patching daily. Although the 6-hours occlusion group had had a faster improvement, at the end of 4 months of treatment both groups achieved similar visual acuity (20/30 visual acuity or at least 3 lines of improvement from baseline), with no statistically significant difference between the groups.147 Another ATS evaluated severe amblyopia (20/100 to 20/400), comparing groups using 6 hours of patching with full-time patching. At the end of the treatment period, both groups had good outcomes with an average improvement in visual acuity of 4.8 lines (6 hours) and 4.7 lines (full time) and no statistically significant difference between the groups.148 Higher hours of patching were associated with worse compliance: Only 6% of patients with higher hours of patching complied for the prescribed time.149 These studies provide useful information about the effect on visual acuity of the number of hours that are prescribed, and can be used as a guide to customize patching treatment for each individual patient.3
- Atropine for penalization proved to be as effective as occlusion. Although the occlusion group had a quicker visual acuity improvement, at the end of 6 months of treatment there was an equal improvement of visual acuity for the 2 groups, and it was maintained in long-term follow-up (up to 15 years). In addition to those who used daily atropine, patients who used atropine once a week showed improvement in visual acuity and had better compliance.150
- Treatment of amblyopia is most effective with children under 7 years of age. Children up to 13 years of age showed significant improvement in vision with patching, although the rate of response to treatment may be slower, require a higher dose of patching, and the extent of recovery may be less complete.151
- There is a high rate of recurrence after the end of amblyopia treatment with similar rates for occlusion and atropine (approximately 25%). This rate was 4 times higher in children who did not have a gradual taper of their treatment for at least 5 weeks following the resolution of amblyopia. Factors also linked with greater recurrence rates included better visual acuity at the end of treatment, greater number of lines of improvement, and previous history of recurrence.152,153
- Children patching with near work for part of the patching time had more improvement than children who patched with no near work as part of the patching regimen.154,155
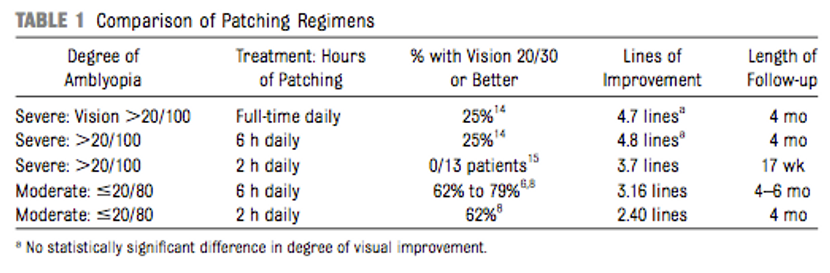
Table 1. Gunton, K. B. (2013) Advances in amblyopia: What have we learned from PEDIG trials? Pediatrics, 131, 540-547.
New Perspectives in Amblyopia
The study of amblyopia over the years has allowed better understanding of brain function. The anatomical and functional structure of the visual system has been studied in further detail, through new models and with more advanced technology, attempting to correlate findings with electrophysiological data, psychophysical data, and now neuroimaging data.
Since Hubel and Wiesel demonstrated anatomical and functional alterations in the primary visual cortex due to amblyopia in animal models, much has been discovered about the impact of amblyopia on the visual system and the importance of a critical period of cerebral plasticity on the effective treatment period. Two major shifts in paradigm regarding amblyopia that these bodies of work brought were the belief that successful treatment of amblyopia outside the critical period is possible, and the concept that amblyopia is more of a binocular, rather than a monocular, disease.156
Treatment of amblyopia outside the critical period
We know that the young brain is more plastic than an adult brain, but we also know that the adult brain is still capable of learning and recovering after injury, so it is clear that there is plasticity at a synaptic level, a cellular level, and at the level of cortical representation. One interpretation in this context is that the critical period ends with an increased threshold for plasticity rather than complete closure, so it is necessary to find stimuli and ways to stimulate the specific plasticity of the adult brain.15,156 Intracortical inhibitory circuitry was discovered as a key factor for defining the limits of cortical plasticity. It has been shown that a brief reduction of gamma-aminobutyric acid-ergic (GABAergic) inhibition in the brains of rats is able to reopen a window of plasticity in the visual system well after the normal closure of the critical periods,157 so several intrinsic and extrinsic modes of augmentation of plasticity have been employed to facilitate amblyopia therapy beyond the critical period of development.
Intrinsic augmentation can be achieved by manipulating the neurotransmitter systems that regulate synaptic plasticity in an environmental and/or behavioral way. This effect is achieved by action of cholinergic pathways and also action of norepinephrine and serotonin to disinhibit cortical visual neurons. One can stimulate this system through environmental enrichment (exercise and visual enrichment), prolonged dark exposure, caloric restriction, and with new or challenging visual tasks (perceptual learning).15,158-162
Extrinsic augmentation uses exogenous manipulation of this endogenous neuromodulatory system. One of these methods is pharmacological and the most commonly used drug for this purpose is levodopa. Non-randomized studies have suggested that the use of levodopa along with occlusive treatment led to improvement in visual acuity, mainly in those amblyopic patients for whom traditional treatment had failed. However, a randomized, placebo-controlled clinical trial conducted by PEDIG showed that the improvement in visual acuity with levodopa did not have a statistically significant difference compared to a placebo, and the improvement in vision in the levodopa group was not sustained during follow-up after stopping the medication.163
Another possibility would be the use of medications that alter the expression of genes to remove the molecular “brakes” on cortical plasticity. Animal models support the use of histone deacetylase inhibitors (valproate) to treat amblyopia.164-167
The neuromodulatory systems can also be accessed via direct and non-invasive activation by subthreshold electric current or transmagnetic stimulation. Transcranial direct current stimulation (TDCS) and transcranial magnetic stimulation (TMS) have been employed in an effort to facilitate plasticity in stroke patients as well as patients with amblyopia. Although both techniques have shown improved contrast sensitivity in amblyopic patients and facilitated stereopsis, the effects were not clinically significant. Further studies are needed to evaluate the efficacy and safety of these technologies.168
Amblyopia as a binocular disease
Amblyopia typically affects visual acuity in one eye, and was always considered a monocular disease. For this reason, the main treatment has been occlusion of the fellow eye to improve the monocular function of the amblyopic eye. However, there are now a large number of studies showing that the deficit in amblyopia extends beyond monocular visual acuity impairment and into higher-order function such as binocular vision, fixation instability, and visuomotor activities due to abnormal interocular interactions.11,169,170 The common factors in those additional deficits in amblyopia are that they are not acuity-limited tasks; they require integration of information over relatively large regions of space and/or time, and they involve extracting a signal from noise.86 These deficits are not corrected by monocular treatment and remain even when visual acuity is recovered after patching.
Based on these findings, it has been argued that amblyopia is intrinsically a binocular problem and that suppression should be addressed first in treatment of amblyopia, as opposed to hoping that binocular vision will return after monocular acuity improvement as result of occlusion therapy. Based on this suggestion, new binocular treatments have been proposed. Hess, Mansouri, and Thompson proposed a treatment based on strengthening binocular combination through a gradual reduction in suppression.57,171,172 Using this binocular approach, they demonstrated that individuals with strabismic amblyopia could combine information normally between their eyes when suppression was reduced by presenting stimuli of different contrasts to each eye via dichoptic viewing. By gradually increasing the contrast presented to the fellow eye, they showed that this approach led to improvement in binocular vision. Eventually, binocular combination occurred when the eyes viewed objects of the same physical contrast. In addition, concomitant improvement in stereopsis and monocular acuity of the amblyopic eye also occurred.6
Based on these findings, these authors proposed a new type of treatment for amblyopia, commonly called dichoptic treatment. It is a strategy that aims to stimulate the 2 eyes simultaneously, thus promoting the possibility of improvement of monocular visual acuity of the amblyopic eye, but also combatting suppression and working to normalize binocular interactions for recovery of binocular vision. To achieve this, the contrast or luminance of visual input to the fellow eye is reduced to match the performance of the amblyopic eye.
This concept has been applied to passive and active forms of training for amblyopia. Passive training modalities include watching movies under dichoptic viewing conditions, allowing each image to be manipulated and simultaneously presented to the 2 eyes independently.58 Active training applies perceptual learning using hand-held tablets which, when combined with red-green glasses, present video games that require binocular function to complete the game’s objective.59,173-175 Both active and passive strategies of dichoptic treatment had good results, with improvement of visual acuity and many cases of normalization or recovery of binocular vision, including in adult individuals.
Given these promising results, PEDIG conducted a large, randomized, controlled trial to compare 1 hour of daily falling-blocks game play with 2 hours of daily patching over 16 weeks between patients from 5 to 13 years old. The study showed a poor adherence to the game regimen prescribed (only 22% of children completing at least 75% of the prescribed play) and found that, for this particular game, the VA improvement was not as good as with 2 hours of prescribed daily patching.176 Similar results were found in another well-designed multi-center randomized clinical trial (BRAVO study).177
Even with these disappointing results, the study authors encourage new research using more engaging gameplay to reduce noncompliance due to the nature of the game itself: The falling-blocks-style game is not appealing to children. New protocols with different and more engaging games such as action-oriented adventure games, first-person shooter games, virtual reality, and 3-dimensional gaming platforms are being analyzed for this purpose.178-180
Although dichoptic treatment did not show substantial improvement in visual acuity and stereopsis, all protocols showed improved contrast processing during the games, which suggests better binocular interaction and decreased suppression. In order to assess the subjective perception of individuals about changes in their vision, it is necessary to evaluate how other visual functions that depend directly on the normal binocular interaction, such as Vernier acuity, contrast sensitivity of different levels of complexity, global movement tasks, fixation stability, and even quality of life (as determined through questionnaires), are improved by dichoptic treatment.
More careful and global study of amblyopic subjects can give us explanations about the great variability of response to treatment of these individuals. It can also help us better define, understand, and categorize amblyopia and thus prepare a more customized treatment for each patient.181
Conclusion
Recent research in amblyopia brings new concepts and a better understanding about this common vision-threatening clinical condition. Now we know that the primary dysfunction within the amblyopic visual system first occurs in area V1, and that the effect caused by amblyopia can be amplified in higher areas of processing. We know that there are significant clinical and functional differences in the patterns of visual loss among the clinically defined categories of amblyopia. Finally, we understand that there appears to be substantial neural plasticity in the amblyopic brain beyond the “critical period,” potentially opening the door to treatment of amblyopia in the teens and into adulthood.
References
- Von Noorden G, Campos E. Binocular Vision and Ocular Motility. 6 eds. St. Louis, Missouri: Mosby, Inc; 2002.
- Zhao PF, Zhou YH, Wang NL, Zhang J. Study of the wavefront aberrations in children with amblyopia. Chin Med J (Engl). 2010;123(11):1431-1435.
- Gunton KB. Advances in amblyopia: What have we learned from PEDIG trials? Pediatrics. 2013;131(3):540-547.
- Billson FA, Fitzgerald BA, Provis JM. Visual deprivation in infancy and childhood: clinical aspects. Aust N Z J Ophthalmol. 1985;13(3):279-286.
- Carlton J, Kaltenthaler E. Amblyopia and quality of life: A systematic review. Eye. 2011;25(4):403-413.
- van De Graaf ES, Van Der Sterre GW, Polling JR, Van Kempen H, Simonsz B, Simonsz HJ. Amblyopia & strabismus questionnaire: Design and initial validation. Strabismus. 2004;12(3):181-193.
- Wong AM. New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Can J Ophthalmol. 2012;47(5):399-409.
- Webber AL. The functional impact of amblyopia. Clin Exp Optom. 2018;101(4):443-450.
- Fawcett SL, Birch EE. Motion VEPs, stereopsis, and bifoveal fusion in children with strabismus. Invest Ophthalmol Vis Sci. 2000;41(2):411-416.
- Fielder AR, Moseley MJ. Does stereopsis matter in humans? Eye (Lond). 1996;10 ( Pt 2):233-238.
- Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013;33(1):67-84.
- Sireteanu R. Binocular luminance summation in humans with defective binocular vision. Invest Ophthalmol Vis Sci. 1987;28(2):349-355.
- Hamm LM, Black J, Dai S, Thompson B. Global processing in amblyopia: A review. Front Psychol. 2014;5(JUN)583.
- Wong EH, Levi DM, McGraw PV. Spatial interactions reveal inhibitory cortical networks in human amblyopia. Vision Res. 2005;45(21):2810-2819.
- Levi DM. Prentice award lecture 2011: Removing the brakes on plasticity in the amblyopic brain. Optom Vis Sci. 2012;89(6):827-838.
- Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Differential changes of magnocellular and parvocellular visual function in early- and late-onset strabismic amblyopia. Invest Ophthalmol Vis Sci. 2006;47(11):4836-4841.
- Sloper J. The other side of amblyopia. J AAPOS. 2016;20(1):1.e-13.
- Hubel DH, Wiesel TN. Effects of monocular deprivation in kittens. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964;248:492-497.
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206(2):419-436.
- Le Vay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191(1):1-51.
- Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Differential changes in color and motion-onset visual evoked potentials from both eyes in early- and late-onset strabismic amblyopia. Invest Ophthalmol Vis Sci. 2008;49(10):4418-4426.
- Cadet N, Huang PC, Superstein R, Koenekoop R, Hess RF. The effects of the age of onset of strabismus on monocular and binocular visual function in genetically identical twins. Can J Ophthalmol. 2018;53(6):609-613.
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol. 1963;26:978-993.
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28(6):1029-1040.
- Tychsen L. Causing and curing infantile esotropia in primates: the role of decorrelated binocular input (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007;105:564-593.
- Tychsen L, Richards M, Wong AM, Demer J, Bradley D, Burkhalter A, et al. Decorrelation of cerebral visual inputs as the sufficient cause of infantile esotropia. Am Orthopt J. 2008;58:60-69.
- Tychsen L, Richards M, Wong A, Foeller P, Burhkalter A, Narasimhan A, et al. Spectrum of infantile esotropia in primates: Behavior, brains, and orbits. J AAPOS. 2008;12(4):375-380.
- Adams DL, Horton JC. Ocular dominance columns: enigmas and challenges. Neuroscientist. 2009;15(1):62-77.
- Headon MP, Sloper JJ, Hiorns RW, Powell TP. Shrinkage of cells in undeprived laminae of the monkey lateral geniculate nucleus following late closure of one eye. Brain Res. 1981;229(1):187-92.
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278(961):377-409.
- von Noorden GK. Histological studies of the visual system in monkeys with experimental amblyopia. Invest Ophthalmol. 1973;12(10):727-738.
- Headon MP, Sloper JJ, Hiorns RW, Powell TP. Effects of monocular closure at different ages on deprived and undeprived cells in the primate lateral geniculate nucleus. Brain Res. 1985;18(1-2):57-78.
- Marg E. Prentice-Memorial Lecture: Is the animal model for stimulus deprivation amblyopia in children valid or useful? Am J Optom Physiol Opt. 1982;59(6):451-464.
- Barrett BT, Bradley A, McGraw PV. Understanding the neural basis of amblyopia. Neuroscientist. 2004;10(2):106-117.
- Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol. 2005;46(3):163-183.
- Ellemberg D, Lewis TL, Maurer D, Brent HP. Influence of monocular deprivation during infancy on the later development of spatial and temporal vision. Vision Res. 2000;40(23):3283-3295.
- Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996;37(8):1532-1538.
- Hamm L, Chen Z, Li J, Black J, Dai S, Yuan J, et al. Interocular suppression in children with deprivation amblyopia. Vision Res. 2017;133:112-120.
- Hamm LM, Chen Z, Li J, Dai S, Black J, Yuan J, et al. Contrast-balanced binocular treatment in children with deprivation amblyopia. Clin Exp Optom. 2018;101(4):541-552.
- DK P. Anisometropia. Brookman KE. Boston: Butterman-Heinemann; 1996:99-121.
- de Vries J. Anisometropia in children: analysis of a hospital population. Br J Ophthalmol. 1985;69(7):504-507.
- Guzowski M, Fraser-Bell S, Rochtchina E, Wang JJ, Mitchell P. Asymmetric refraction in an older population: the Blue Mountains Eye Study. Am J Ophthalmol. 2003;136(3):551-553.
- Huynh SC, Wang XY, Ip J, Robaei D, Kifley A, Rose KA, et al. Prevalence and associations of anisometropia and anisoastigmatism in a population based sample of 6 year old children. Br J Ophthal. 2006;90(5):597-601.
- O'Donoghue L, McClelland JF, Logan NS, Rudnicka AR, Owen CG, Saunders KJ. Profile of anisometropia and aniso-astigmatism in children: prevalence and association with age, ocular biometric measures, and refractive status. Invest Ophthalmol Vis Sci. 2013;54(1):602-608.
- Copps LA. Vision in Anisometropia*. Am J Ophthalmology. 1944;27(6):641-644.
- Toor S, Horwood AM, Riddell P. Asymmetrical accommodation in hyperopic anisometropic amblyopia. Br J Ophthalmol. 2018;102(6):772-778.
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003;3(5):380-405.
- Levi DM, McKee SP, Movshon JA. Visual deficits in anisometropia. Vision Res. 2011;51(1):48-57.
- Sengpiel F, Troilo D, Kind PC, Graham B, Blakemore C. Functional architecture of area 17 in normal and monocularly deprived marmosets (Callithrix jacchus). Vis Neurosci. 1996;13(1):145-160.
- Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque's visual system. III. Physiological observations. J Neurosci. 1987;7(5):1340-1351.
- Helveston EM. Relationship between degree of anisometropia and depth of amblyopia. Am J Ophthalmol. 1966;62(4):757-759.
- Harrad RA, Hess RF. Binocular integration of contrast information in amblyopia. Vision Res. 1992;32(11):2135-2150.
- Harrad R. Psychophysics of suppression. Eye (Lond). 1996;10 ( Pt 2):270-273.
- Muckli L, Kiess S, Tonhausen N, Singer W, Goebel R, Sireteanu R. Cerebral correlates of impaired grating perception in individual, psychophysically assessed human amblyopes. Vision Res. 2006;46(4):506-526.
- Weakley DR Jr. The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108(1):163-171.
- Cotter SA, Edwards AR, Wallace DK, Beck RW, Arnold RW, Astle WF, et al; Pediatric Eye Disease Investigator Group. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113(6):895-903.
- Hess RF, Mansouri B, Thompson B. Restoration of binocular vision in amblyopia. Strabismus. 2011;19(3):110-118.
- Li SL, Reynaud A, Hess RF, Wang YZ, Jost RM, Morale SE, et al. Dichoptic movie viewing treats childhood amblyopia. J AAPOS. 2015;19(5):401-405.
- Birch EE, Li SL, Jost RM, Morale SE, De La Cruz A, Stager D, et al. Binocular iPad treatment for amblyopia in preschool children. J AAPOS. 2015;19(1):6-11.
- Baker DH, Meese TS, Mansouri B, Hess RF. Binocular summation of contrast remains intact in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2007;48(11):5332-5338.
- Kiorpes L, Kiper DC, O'Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998;18(16):6411-6424.
- Smith DC. Developmental alterations in binocular competitive interactions and visual acuity in visually deprived cats. J Comp Neurol. 1981;198(4):667-676.
- Crawford ML, von Noorden GK. Concomitant strabismus and cortical eye dominance in young rhesus monkeys. Trans Ophthalmol Soc U K. 1979;99(3):369-374.
- Crawford ML, Harwerth RS. Ocular dominance column width and contrast sensitivity in monkeys reared with strabismus or anisometropia. Invest Ophthalmol Vis Sci. 2004;45(9):3036-3042.
- Tychsen L, Wong AM, Burkhalter A. Paucity of horizontal connections for binocular vision in V1 of naturally strabismic macaques: Cytochrome oxidase compartment specificity. J Comp Neurol. 2004;474(2):261-275.
- Sengpiel F, Blakemore C, Harrad R. Interocular suppression in the primary visual cortex: a possible neural basis of binocular rivalry. Vision Res. 1995;35(2):179-195.
- Sengpiel F, Blakemore C. The neural basis of suppression and amblyopia in strabismus. Eye. 1996;10(2):250-258.
- Smith EL 3rd, Chino YM, Ni J, Cheng H, Crawford ML, Harwerth RS. Residual binocular interactions in the striate cortex of monkeys reared with abnormal binocular vision. J Neurophysiol. 1997;78(3):1353-1362.
- Hess RF, Campbell FW, Greenhalgh T. On the nature of the neural abnormality in human amblyopia; neural aberrations and neural sensitivity loss. Pflügers Arch. 1978;377(3):201-207.
- Hess RF, Holliday IE. The spatial localization deficit in amblyopia. Vision Res. 1992;32(7):1319-1339.
- Hess RF, Wang YZ, Demanins R, Wilkinson F, Wilson HR. A deficit in strabismic amblyopia for global shape detection. Vision Res. 1999;39(5):901-914.
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Res. 1985;25(7):979-991.
- Bonneh YS, Sagi D, Polat U. Spatial and temporal crowding in amblyopia. Vision Res. 2007;47(14):1950-1962.
- Chung ST, Li RW, Levi DM. Crowding between first- and second-order letters in amblyopia. Vision Res. 2008;48(6):788-798.
- Hubel DH, Wiesel TN. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965;28(6):1041-1059.
- Barlow HB, Blakemore C, Pettigrew JD. The neural mechanism of binocular depth discrimination. J Physiol. 1967;193(2):327-342.
- Blakemore C. The conditions required for the maintenance of binocularity in the kitten's visual cortex. J Physiol. 1976;261(2):423-444.
- Hoyt CS. Amblyopia: a neuro-ophthalmic view. J Neuroophthalmol. 2005;25(3):227-231.
- Norcia AM, Hale J, Pettet MW, McKee SP, Harrad RA. Disparity tuning of binocular facilitation and suppression after normal versus abnormal visual development. Invest Ophthalmol Vis Sci. 2009;50(3):1168-1175.
- O'Connor AR, Birch EE, Anderson S, Draper H. Relationship between binocular vision, visual acuity, and fine motor skills. Optom Vis Sci. 2010;87(12):942-947.
- Zipori AB, Colpa L, Wong AMF, Cushing SL, Gordon KA. Postural stability and visual impairment: Assessing balance in children with strabismus and amblyopia. PLoS One. 2018;13(10):e0205857.
- Hess RF, Bradley A. Contrast perception above threshold is only minimally impaired in human amblyopia. Nature. 1980;287(5781):463-464.
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299(5884):583-591.
- Movshon JA. Cortical effects of monocular deprivation: suppression or deafferentation? Nature. 1981;291(5813):284-285.
- Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Curr Opin Neurobiol. 1999;9(4):480-486.
- Kiorpes L. Visual processing in amblyopia: animal studies. Strabismus. 2006;14(1):3-10.
- Horton JC, Hocking DR. Pattern of ocular dominance columns in human striate cortex in strabismic amblyopia. Vis Neurosci. 1996;13(4):787-795.
- Bi H, Zhang B, Tao X, Harwerth RS, Smith EL 3rd, Chino YM. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cereb Cortex. 2011;21(9):2033-2045.
- Simmers AJ, Ledgeway T, Hess RF. The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Res. 2005;45(4):449-460.
- Levi DM, Waugh SJ, Beard BL. Spatial scale shifts in amblyopia. Vision Res. 1994;34(24):3315-3333.
- Levi DM, Yu C, Kuai SG, Rislove E. Global contour processing in amblyopia. Vision Res. 2007;47(4):512-524.
- Polat U, Sagi D, Norcia AM. Abnormal long-range spatial interactions in amblyopia. Vision Res. 1997;37(6):737-744.
- Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Res. 1977;17(9):1049-1055.
- Hou C, Good WV, Norcia AM. Validation study of VEP vernier acuity in normal-vision and amblyopic adults. Invest Ophthalmol Vis Sci. 2007;48(9):4070-4078.
- Sharma V, Levi DM, Klein SA. Undercounting features and missing features: evidence for a high-level deficit in strabismic amblyopia. Nat Neurosci. 2000;3(5):496-501.
- Levi DM. Crowding-An essential bottleneck for object recognition: A mini-review. Vision Res. 2008;48(5):635-654.
- Popple AV, Levi DM. The attentional blink in amblyopia. J Vis. 2008;8(13):12.1-9.
- Kanonidou E, Proudlock FA, Gottlob I. Reading strategies in mild to moderate strabismic amblyopia: an eye movement investigation. Invest Ophthalmol Vis Sci. 2010;51(7):3502-3508.
- Kugathasan L, Partanen M, Chu V, Lyons C, Giaschi D. Reading ability of children treated for amblyopia. Vision Res. 2019;156:28-38.
- Birch EE, Castañeda YS, Cheng-Patel CS, Morale SE, Kelly KR, Beauchamp CL, et al. Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. JAMA Ophthalmology. 2019;137(2):167-173.
- Farzin F, Norcia AM. Impaired visual decision-making in individuals with amblyopia. J Vis. 2011;11(14).
- Ho CS, Paul PS, Asirvatham A, Cavanagh P, Cline R, Giaschi DE. Abnormal spatial selection and tracking in children with amblyopia. Vision Res. 2006;46(19):3274-3283.
- Richards MD, Goltz HC, Wong AMF. Audiovisual perception in amblyopia: A review and synthesis. Exp Eye Res. 2018 May 17 [Epub ahead of print].
- Burgmeier R, Desai RU, Farner KC, Tiano B, Lacey R, Volpe NJ, et al. The effect of amblyopia on visual-auditory speech perception: why mothers may say "Look at me when I'm talking to you". JAMA Ophthalmology. 2015;133(1):11-16.
- Narinesingh C, Goltz HC, Raashid RA, Wong AM. Developmental trajectory of McGurk effect susceptibility in children and adults with amblyopia. Invest Ophthalmol Vis Sci. 2015;56(3):2107-2113.
- Putzar L, Goerendt I, Heed T, Richard G, Büchel C, Röder B. The neural basis of lip-reading capabilities is altered by early visual deprivation. Neuropsychologia. 2010;48(7):2158-2166.
- Richards MD, Goltz HC, Wong AME. Optimal audiovisual integration in the ventriloquism effect but pervasive deficits in unisensory spatial localization in amblyopia. Invest Ophthalmol Vis Sci. 2018;59(1):122-131.
- Richards MD, Goltz HC, Wong AMF. Alterations in audiovisual simultaneity perception in amblyopia. PLoS One. 2017;12(6):e0179516.
- Mansouri B, Allen HA, Hess RF. Detection, discrimination and integration of second-order orientation information in strabismic and anisometropic amblyopia. Vision Res. 2005;45(18):2449-2460.
- Wong EH, Levi DM, McGraw PV. Is second-order spatial loss in amblyopia explained by the loss of first-order spatial input? Vision Res. 2001;41(23):2951-2960.
- Kiorpes L, Tang C, Movshon JA. Sensitivity to visual motion in amblyopic macaque monkeys. Vis Neurosci. 2006;23(2):247-256.
- Hayward J, Truong G, Partanen M, Giaschi D. Effects of speed, age, and amblyopia on the perception of motion-defined form. Vision Res. 2011;51(20):2216-2223.
- Meier K, Giaschi D. Unilateral amblyopia affects two eyes: fellow eye deficits in amblyopia. Invest Ophthalmol Vis Sci. 2017;58(3):1779-1800.
- Mirabella G, Hay S, Wong AM. Deficits in perception of images of real-world scenes in patients with a history of amblyopia. Arch Ophthalmol. 2011;129(2):176-183.
- Thompson B, Richard A, Churan J, Hess RF, Aaen-Stockdale C, Pack CC. Impaired spatial and binocular summation for motion direction discrimination in strabismic amblyopia. Vision Res. 2011;51(6):577-584.
- Mansouri B, Hess RF. The global processing deficit in amblyopia involves noise segregation. Vision Res. 2006;46(24):4104-4117.
- Levi DM, Klein SA, Sharma V. Position jitter and undersampling in pattern perception. Vision Res. 1999;39(3):445-465.
- Norcia AM, Sampath V, Hou C, Pettet MW. Experience-expectant development of contour integration mechanisms in human visual cortex. J Vis. 2005;5(2):116-130.
- Popple AV, Levi DM. Amblyopes see true alignment where normal observers see illusory tilt. Proc Natl Acad Sci U S A. 2000;97(21):11667-11672.
- Demer JL, Grafton S, Marg E, Mazziotta JC, Nuwer M. Positron-emission tomographic study of human amblyopia with use of defined visual stimuli. J AAPOS. 1997;1(3):158-171.
- Choi MY, Lee DS, Hwang JM, Choi DG, Lee KM, Park KH, et al. Characteristics of glucose metabolism in the visual cortex of amblyopes using positron-emission tomography and statistical parametric mapping. J Pediatr Ophthalmol Strabismus. 2002;39(1):11-19.
- Joly O, Frankó E. Neuroimaging of amblyopia and binocular vision: a review. Front in Integr Neurosci. 2014 Aug6;8:62.
- Barnes GR, Hess RF, Dumoulin SO, Achtman RL, Pike GB. The cortical deficit in humans with strabismic amblyopia. J Physiol. 2001;533(Pt 1):281-297.
- Li X, Dumoulin SO, Mansouri B, Hess RF. Cortical deficits in human amblyopia: Their regional distribution and their relationship to the contrast detection deficit. Invest Ophthalmol Visl Sci. 2007;48(4):1575-1591.
- Goodyear BG, Nicolle DA, Menon RS. High resolution fMRI of ocular dominance columns within the visual cortex of human amblyopes. Strabismus. 2002;10(2):129-136.
- Hess RF, Thompson B, Gole GA, Mullen KT. The amblyopic deficit and its relationship to geniculo-cortical processing streams. J Neurophysiol. 2010;104(1):475-483.
- Lv B, He H, Li X, Zhang Z, Huang W, Li M, et al. Structural and functional deficits in human amblyopia. Neurosci Lett. 2008;437(1):5-9.
- Mendola JD, Conner IP, Roy A, Chan ST, Schwartz TL, Odom JV, et al. Voxel-based analysis of MRI detects abnormal visual cortex in children and adults with amblyopia. Hum Brain Mapp. 2005;25(2):222-236.
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255(5043):419-423.
- Costa MF, Cunha G, de Oliveira Marques JP, Castelo-Branco M. Strabismic amblyopia disrupts the hemispheric asymmetry for spatial stimuli in cortical visual processing. The British journal of visual impairment. 2016;34(2):141-50.
- Liang M, Xie B, Yang H, Yin X, Wang H, Yu L, et al. Altered interhemispheric functional connectivity in patients with anisometropic and strabismic amblyopia: a resting-state fMRI study. Neuroradiology. 2017;59(5):517-524.
- Choi MY, Lee KM, Hwang J-M, Choi DG, Lee DS, Park KH, et al. Comparison between anisometropic and strabismic amblyopia using functional magnetic resonance imaging. Br J Ophthalmol. 2001;85(9):1052-1056.
- Mendola JD, Lam J, Rosenstein M, Lewis LB, Shmuel A. Partial correlation analysis reveals abnormal retinotopically organized functional connectivity of visual areas in amblyopia. Neuroimage Clin. 2018;18:192-201.
- Birch EE, Holmes JM. The clinical profile of amblyopia in children younger than 3 years of age. J AAPOS. 2010;14(6):494- 497.
- Salomão SR, Ventura DF. Large sample population age norms for visual acuities obtained with Vistech-Teller Acuity Cards. Invest Ophthalmol Vis Sci. 1995;36(3):657-670.
- Wallace DK, Repka MX, Lee KA, Melia M, Christiansen SP, Morse CL, et al; American Academy of Pediatric Ophthalmology/Strabismus Preferred Practice Pattern Pediatric Ophthalmology Panel. Amblyopia Preferred Practice Pattern®. Ophthalmology. 2018;125(1):P105-P42.
- O'Hara MA. Instrument-based pediatric vision screening. Curr Opin Ophthalmol. 2016;27(5):398-401.
- Williams C, Harrad RA, Harvey I, Sparrow JM, ALSPAC Study Team. Screening for amblyopia in preschool children: results of a population-based, randomised controlled trial. Ophthalmic Epidemiol. 2001;8(5):279-295.
- Cotter SA, Varma R, Tarczy-Hornoch K, McKean-Cowdin R, Lin J, Wen G, et al; Joint Writing Committee for the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study Groups. Risk factors associated with childhood strabismus: the multi-ethnic pediatric eye disease and Baltimore pediatric eye disease studies. Ophthalmology. 2011;118(11):2251-2261.
- Hunter DG, Nassif DS, Piskun NV, Winsor R, Gramatikov BI, Guyton DL. Pediatric Vision Screener 1: instrument design and operation. J Biomed Opt. 2004;9(6):1363-1368.
- AAPOS CsEFo. 2019. Screen eyes early (SEE) in the medical home. Available from: https://www.childrenseyefoundation.org/see/.
- PEDIG Amblyopia Treatment Study Index [Available from: https://public.jaeb.org/pedig/pubs.]
- PEDIG. Pediatric Eye Disease Investigator Group 2019 [Available from: https://public.jaeb.org/pedig/pubs.]
- Stewart CE, Fielder AR, Stephens DA, Moseley MJ. Design of the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Br J Ophthalmol. 2002;86(8):915-919.
- Cotter SA, Foster NC, Holmes JM, Melia BM, Wallace DK, Repka MX, et al; Writing Committee for the Pediatric Eye Disease Investigator Group. Optical treatment of strabismic and combined strabismic-anisometropic amblyopia. Ophthalmology. 2012;119(1):150-158.
- Wallace DK, Edwards AR, Cotter SA, Beck RW, Arnold RW, Astle WF, et al; Pediatric Eye Disease Investigator Group. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113(6):904-912.
- Repka MX, Beck RW, Holmes JM, Birch EE, Chandler DL, Cotter SA, et al; Pediatric Eye Disease Investigator Group. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol. 2003;121(5):603-611.
- Holmes JM, Kraker RT, Beck RW, Birch EE, Cotter SA, Everett DF, et al; Pediatric Eye Disease Investigator Group. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110(11):2075-2087.
- Gottlob I, Awan M, Proudlock F. The role of compliance in 2 vs 6 hours of patching in children with amblyopia. Arch Ophthalmol. 2004;122(3):422-423; author reply 4-5.
- Repka MX, Kraker RT, Holmes JM, Summers AI, Glaser SR, Barnhardt CN, et al; Pediatric Eye Disease Investigator Group. Atropine vs patching for treatment of moderate amblyopia: follow-up at 15 years of age of a randomized clinical trial. JAMA Ophthalmol. 2014;132(7):799-805.
- Holmes JM, Lazar EL, Melia BM, Astle WF, Dagi LR, Donahue SP, et al; Pediatric Eye Disease Investigator Group. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol. 2011;129(11):1451-1457.
- Holmes JM, Melia M, Bradfield YS, Cruz OA, Forbes B, Pediatric Eye Disease Investigator Group. Factors associated with recurrence of amblyopia on cessation of patching. Ophthalmology. 2007;114(8):1427-1432.
- Birch EE, Fawcett SL, Morale SE, Weakley DR Jr, Wheaton DH. Risk factors for accommodative esotropia among hypermetropic children. Invest Ophthalmol Vis Sci. 2005;46(2):526-529.
- Pediatric Eye Disease Investigator Group. A randomized trial of near versus distance activities while patching for amblyopia in children aged 3 to less than 7 years. Ophthalmology. 2008;115(11):2071-2078.
- Holmes JM, Edwards AR, Beck RW, Arnold RW, Johnson DA, Klimek DL, et al; Pediatric Eye Disease Investigator Group. A randomized pilot study of near activities versus non-near activities during patching therapy for amblyopia. J AAPOS. 2005;9(2):129-136.
- Gaier ED, Hunter DG. Advances in amblyopia treatment: paradigm shifts and future directions. Int Ophthalmol Clin. 2017;57(4):117-128.
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, et al. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010;30(1):361-371.
- Baroncelli L, Bonaccorsi J, Milanese M, Bonifacino T, Giribaldi F, Manno I, et al. Enriched experience and recovery from amblyopia in adult rats: impact of motor, social and sensory components. Neuropharmacology. 2012;62(7):2388-2397.
- Kaneko M, Stryker MP. Sensory experience during locomotion promotes recovery of function in adult visual cortex. Elife. 2014;3:e02798.
- Imamura K, Kasamatsu T. Interaction of noradrenergic and cholinergic systems in regulation of ocular dominance plasticity. Neurosci Res. 1989;6(6):519-536.
- Duffy KR, Mitchell DE. Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Curr Biol. 2013;23(5):382-386.
- Spolidoro M, Baroncelli L, Putignano E, Maya-Vetencourt JF, Viegi A, Maffei L. Food restriction enhances visual cortex plasticity in adulthood. Nat Commun. 2011;2:320.
- Repka MX, Kraker RT, Dean TW, Beck RW, Siatkowski RM, Holmes JM, et al; Pediatric Eye Disease Investigator Group. A randomized trial of levodopa as treatment for residual amblyopia in older children. Ophthalmology. 2015;122(5):874-881.
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18(1):101-107.
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30(45):14964-14971.
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, et al. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53(5):747-759.
- Baroncelli L, Scali M, Sansevero G, Olimpico F, Manno I, Costa M, et al. Experience affects critical period plasticity in the visual cortex through an epigenetic regulation of histone post-translational modifications. J Neurosci. 2016;36(12):3430-3440.
- Thompson B, Mansouri B, Koski L, Hess RF. From motor cortex to visual cortex: the application of noninvasive brain stimulation to amblyopia. Dev Psychobiol. 2012;54(3):263-273.
- Levi DM, Knill DC, Bavelier D. Stereopsis and amblyopia: A mini-review. Vision Res. 2015;114:17-30.
- Zhao W, Jia WL, Chen G, Luo Y, Lin B, He Q, et al. A complete investigation of monocular and binocular functions in clinically treated amblyopia. Sci Rep. 2017;7(1):10682.
- Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: antisuppression therapy. Optom Vis Sci. 2010;87(9):697-704.
- Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restor Neurol Neurosci. 2010;28(6):793-802.
- Hess RF, Thompson B. Amblyopia and the binocular approach to its therapy. Vision Res. 2015;114:4-16.
- Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol. 2013;23(8):R308-9.
- Li SL, Jost RM, Morale SE, Stager DR, Dao L, Stager D, et al. A binocular iPad treatment for amblyopic children. Eye (Lond). 2014;28(10):1246-1253.
- Holmes JM, Manh VM, Lazar EL, Beck RW, Birch EE, Kraker RT, et al. Effect of a binocular iPad game vs part-time patching in children aged 5 to 12 years with amblyopia: a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1391-1400.
- Gao TY, Guo CX, Babu RJ, Black JM, Bobier WR, Chakraborty A, et al; BRAVO Study Team. Effectiveness of a binocular video game vs placebo video game for improving visual functions in older children, teenagers, and adults with amblyopia: a randomized clinical trial. JAMA Ophthalmol. 2018;136(2):172-181.
- Kelly KR, Jost RM, Dao L, Beauchamp CL, Leffler JN, Birch EE. Binocular iPad game vs patching for treatment of amblyopia in children: a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1402-1408.
- Vedamurthy I, Knill DC, Huang SJ, Yung A, Ding J, Kwon OS, et al. Recovering stereo vision by squashing virtual bugs in a virtual reality environment. Philos Trans R Soc Lond B Biol Sci. 2016;371(1697).
- Žiak P, Holm A, Halička J, Mojžiš P, Piñero DP. Amblyopia treatment of adults with dichoptic training using the virtual reality oculus rift head mounted display: preliminary results. BMC Ophthalmol. 2017;17(1):105.
- Holmes JM. Lessons from recent randomized clinical trials of binocular treatment for amblyopia. JAMA Ophthalmol. 2018;136(2):181-183.