Disease Entity
Retinoblastoma is a primitive neuroectodermal intraocular malignancy that affects young children. It is the most common primary intraocular malignancy in children, frequently presenting with leukocoria.
Incidence and Epidemiology
Multiple estimates of the incidence of retinoblastoma in various populations and countries have been published. It is the most common intraocular malignancy afflicting children.1 The estimated incidence of retinoblastoma varies by country from 3.4 to 42.6 cases per million live births. In the United States, the incidence is 11.8 cases per million live births among children less than 5 years of age. Retinoblastoma represents 6.1% of all cancers in this age group. There are approximately 350 new cases annually in the United States.
The majority of patients present before 5 years of age, with a median age at diagnosis of 18 months.2 At presentation, approximately two-thirds of cases are unilateral and one-third bilateral. Patients diagnosed with retinoblastoma are categorized by whether the mutation is germline (present in all cells of the body) or somatic (present in the tumor only). Laterality generally, but imperfectly, predicts whether the retinoblastoma is secondary to a germline or somatic mutation. All patients with bilateral disease and 15% of patients with unilateral disease are found to have a germline mutation (see Genetics below). The incidence is equal in males and females. There is no clear racial predilection for retinoblastoma.
Most children with retinoblastoma present with leukocoria (a white pupillary reflex; see Figure 1) or strabismus (a lazy eye).
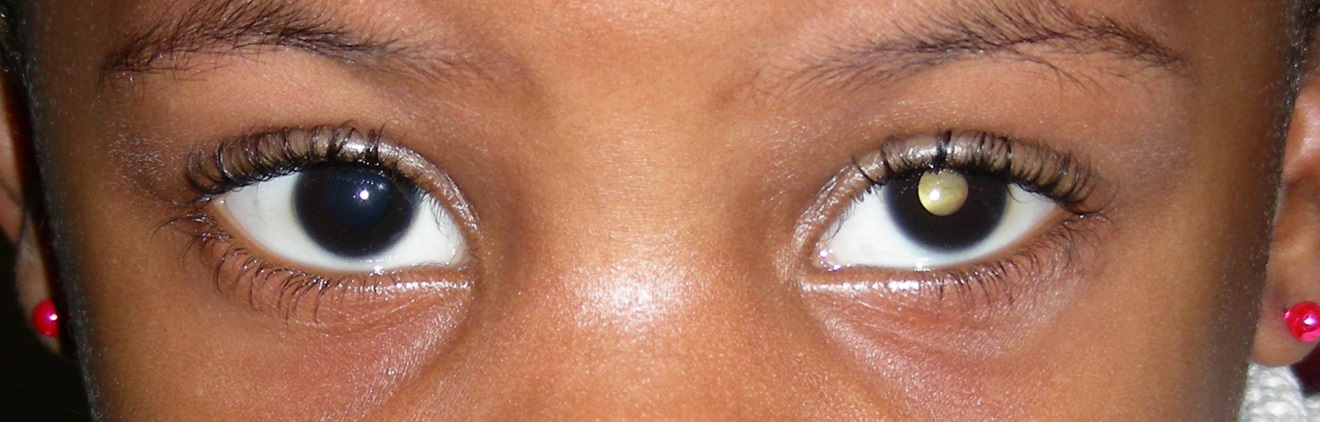
Figure 1. Leukocoria.
Genetics
Due to familial cases of retinoblastoma, it has long been known that there was a genetic underpinning to the development of some forms of this cancer. However, the genetic locus for this tumor was only elucidated in the past few decades.
Knudson described the "two-hit" mechanism for tumorigenesis in patients with retinoblastoma in 1971. The hypothesis was that mutations in both active copies of a gene responsible for normal retinal development are required for the development of retinoblastoma. The initial mutation inactivates one copy of the gene. This mutation can occur in somatic or germline cells. The second mutation occurs in somatic cells in the developing retina.
If the first mutation occurs in germline cells, the patient has the heritable form of retinoblastoma. In 90% of cases, this represents a new mutation without a family history of retinoblastoma. These children present with retinoblastoma at a younger age due to the fact that all the cells already have the "first hit." The timing of tumor development between heritable and nonheritable retinoblastoma was an early clue for Knudson's hypothesis.
In 1984, the gene required for the development of retinoblastoma was mapped to a single locus by Murphree via linkage studies due to the association between 13q- syndrome and retinoblastoma. It was found to be located on sub-band 13q14.2.3
In 1986, the retinoblastoma gene was identified by Friend and termed RB1.4 The retinoblastoma gene was the first described tumor suppressor gene. It restricts uncontrolled passage through the cell cycle, as compared to the classic oncogene, which promotes cell growth. Because RB1 is a tumor suppressor gene, both alleles must be inactivated for tumor development.2
Germline cases, in which all cells in the body carry one mutated copy of the retinoblastoma gene, represent approximately 40% of retinoblastoma cases.2 Patients with germline disease tend to have bilateral and multifocal tumors. Germline patients also have a significantly increased risk for secondary tumors, including primitive neuroectodermal tumors in the brain (so-called "trilateral retinoblastoma"). Finally, there is a 45% chance that their offspring will develop retinoblastoma because this trait is inherited in an autosomal dominant fashion with 90% penetrance.5
If both of the RB gene mutations occur in a somatic cell of the developing retina, it is not heritable, but rather sporadic retinoblastoma, and the patient presents with unilateral disease. Although most unilateral tumors are sporadic and nonhereditary, approximately 15% of unilateral cases are found to have germline mutations.2 Additionally, some children can have unilateral disease at presentation, but subsequently progress to bilateral involvement. Thus, determining whether the initial mutation is germline via serum genetic testing is critically important in the clinical management of these patients. Free genetic testing for retinoblastoma patients to determine the status of the RB1 gene in the germline is available through the eyeGENE program of the National Eye Institute (NEI).
Classification Systems
Several detailed systems can be used to stage retinoblastoma (see below). For practical purposes, when determining the best treatment options, doctors often divide retinoblastomas into 2 main groups: intraocular and extraocular.
Extraocular cancers can be divided further into orbital retinoblastomas, which have spread only to the eye socket, and metastatic retinoblastomas, which have spread to distant parts of the body, such as the brain or bone marrow.
For intraocular retinoblastoma, there are two commonly used classification systems. The Reese-Ellsworth classification system was developed in the 1950s to predict the prognosis for globe salvage after treatment with radiation. It stratifies patients into groups 1 through 5:
- Very favorable
- Solitary tumor < 4 disc diameter (DD) in size, at or behind equator
- Multiple tumors, none more than 4 DD in size, all at or behind equator
- Favorable
- Solitary tumor, 4 to 10 DD in size, at or behind equator
- Multiple tumors, 4 to 10 DD in size, behind equator
- Doubtful
- Any tumor anterior to equator
- Solitary tumor, larger than 10 DD, behind equator
- Unfavorable
- Multiple tumors, some larger than 10 DD in size
- Any lesion extending anteriorly to the ora serrata
- Very unfavorable
- Massive tumor involving more than half the retina
- Vitreous seeding
As treatment modalities evolved, clinicians' preferred treatment modality shifted from external beam radiation to chemotherapy due to documented increased risk of patients developing secondary tumors following radiation. The Reese-Ellsworth classification system no longer accurately reflected prognosis with the newer treatment modalities. Thus, International Classification fo Intraocular Retinoblastoma (ICRB) was developed to better predict those with intraocular retinoblastoma who are likely to be cured (and the eye be saved) without the need for enucleation or external-beam radiation treatment. The ICRB classifies tumors from A through E:
- Small tumors (3 mm or less across) that are only in the retina and are located > 3 mm from foveola > 1.5 mm from the disc
- Tumors larger than 3 mm or in a macular or juxtapapillary location. There can be a cuff of subretinal fluid < 3 mm from the tumor without associated subretinal seeding.
- Tumor with localized subretinal or vitreous seeding (Figure 2) within 3 mm of the tumor and up to 1 quadrant of subretinal fluid
- Tumors with diffuse subretinal or vitreous seeding > 3 mm from tumor and extensive subretinal fluid
- Extensive retinoblastoma with poor prognostic signs including neovascular glaucoma, tumor anterior to the vitreous face (Figure 2), diffuse infiltrating retinoblastoma, phthisis bulbi, aseptic orbital cellulitis, or opaque media from diffuse vitreous hemorrhage
The ICRB has been shown to reliably predict the success of globe salvage in the chemoreduction era as reported on a large series of patients.6 The successful outcome of Group D eyes treated with IV chemotherapy was corroborated by Berry and colleagues.7 However, Group E eyes have a significantly lower success rate.8
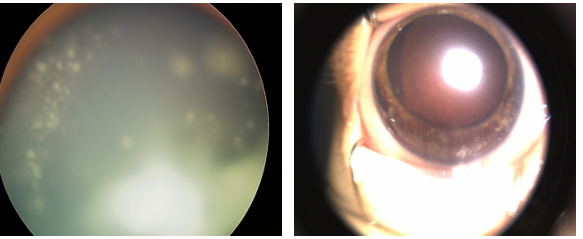
Figure 2. Left: vitreous seeding. Right: anterior segment invasion with nodular iris opacities and diffuse vitreous hemorrhage.
Pathology
Retinoblastoma cells are medium sized (twice the size of lymphocytes), have high nuclear-cytoplasmic ratio, marked apoptotic and mitotic activity, and stain blue with hematoxylin and eosin (H&E) stain. These tumor cells tend to replace the retina and grow around a feeding vessel as a cuff. Once the tumor cells outgrow their vascular supply (approximately 90–100‑μm radius around the feeding vessel), they undergo necrosis with calcification. Necrotic cells appear pink on H&E staining and calcification has a hint of violet or purple. Most tumors tend to grow in sheets or large foci of undifferentiated tumor cells.
More differentiated cells have grouping patterns called rosettes. A ring of cells surrounding an empty lumen is known as a Flexner-Wintersteiner rosette (Figure 3A). A ring of cells with an eosinophilic fibrillary center is called a Homer-Wright rosette (Figure 3B); these are commonly found.
Fleurettes (Figure 3C) are retinoblastoma cells that have undergone greater photoreceptor differentiation and group together as a bouquet. Fleurettes lack mitosis or necrosis. A tumor composed of fleurettes is deemed benign and called retinoma or retinocytoma.
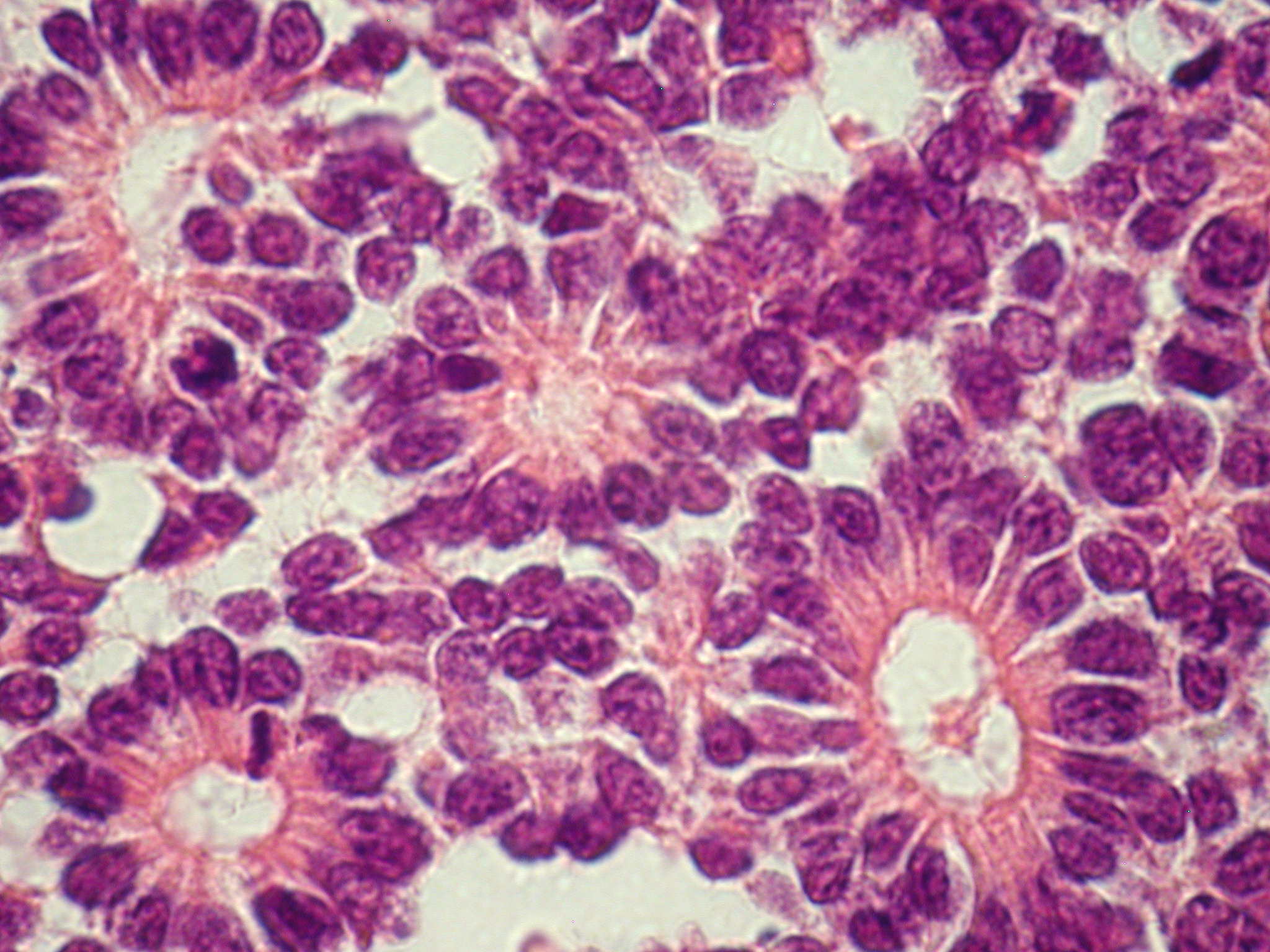
Figure 3A. Flexner-Wintersteiner rosettes.

Figure 3B. Homer Wright rosettes.
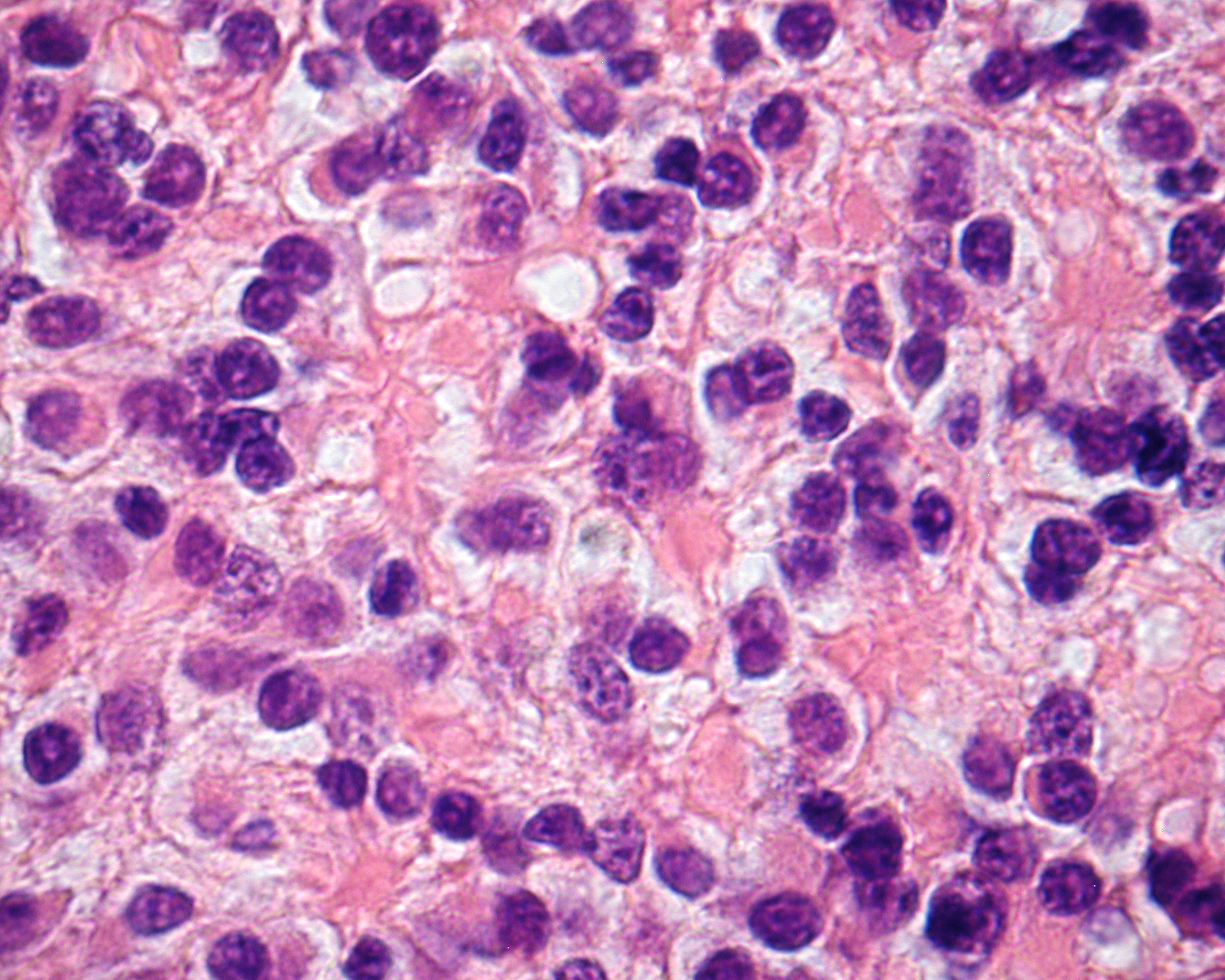
Figure 3C. Fleurettes.
A critical role of pathology in the management of retinoblastoma is the identification of pathologic features that predispose to extraocular relapse. These include invasion into the choroid, the sclera, and/or the optic nerve. Although there is no clear consensus among ocular oncologists, these features are used to decide whether a child will require adjuvant chemotherapy after enucleation to prevent relapse with extraocular disease.9
Diagnosis
Screening
The American Academy of Pediatrics policy statement on Red Reflex Examinations in Neonates, Infants, and Children recommends that all neonates, infants, and children should have an examination of the red reflex before discharge from the neonatal nursery and at all subsequent routine health supervision visits.10 The red reflex test is performed in a dimly lit or dark room with a direct ophthalmoscope or a retinoscope from a distance of about 1–1.5 feet from the patient. Leukocoria is the most common presentation of retinoblastoma, and all infants or children with an abnormal red reflex require immediate referral to an ophthalmologist skilled in pediatric examinations.
Newborn screening by an ophthalmologist is of greatest use with a positive family history of retinoblastoma. Offspring and siblings of affected patients require regular screening examinations in childhood unless genetic testing is done and no retinoblastoma mutation is identified, in which case the risk is similar to that of the general population. Genetic counseling for families with retinoblastoma can help determine whether other family members are at risk for developing disease and the risk to future offspring.
Clinical Presentation
Early diagnosis of retinoblastoma can maximize the patient's visual prognosis as well as survival rate. Retinoblastoma must always be in the differential diagnosis for any child who presents with strabismus, leukocoria, a red eye, or a cellulitis-like picture. Retinoblastoma is the most important differential diagnosis for a child with leukocoria. Leukocoria, or the loss of the normal red reflex, is secondary to the tumor filling the globe. Strabismus can also be a presenting sign of retinoblastoma, so all patients undergoing a strabismus evaluation should have a full dilated ophthalmic examination.
In rare cases, retinoblastoma can present with pain and intraocular inflammation and mimic endophthalmitis, uveitis, vitreous hemorrhage, or preseptal/orbital cellulitis. This can occur with any tumor, but particularly in the diffuse infiltrating form of retinoblastoma. Periorbital inflammation is a poor prognostic sign and can occur when the tumor has spread outside the globe or when a very large tumor undergoes massive necrosis.
Retinoblastoma can demonstrate local invasion into the choroid or sclera or spread along the optic nerve directly into the orbit. It can also metastasize hematogenously to bone, liver, the central nerve system (CNS), and other organs.
History
A careful history of present illness, family history, and thorough ophthalmic examination, as well as appropriate ancillary studies, are critical for prompt diagnosis. The ophthalmologist should specifically inquire about a family history of blindness, eye tumors, childhood malignancies, and enucleations. A family history of other cancers such as sarcomas can also be suggestive. The parent should be asked whether leukocoria or strabismus has been observed, and if so, for what time period.
Physical Examination
A red reflex test to confirm the presence of leukocoria should be done. A complete pediatric ophthalmic examination is then indicated including age-appropriate visual acuity testing and pupillary response; anterior examination to evaluate for proptosis, ciliary injection, pseudohypopyon, and signs of secondary glaucoma; and dilated examination to evaluate for retinal masses. A- and B-scan to look for retinal based masses with intralesional calcification is also critical. Given the age of many of these patients, a detailed exam might not be possible in the office. If there is any suspicion for retinoblastoma, the patient should undergo an examination under anesthesia to confirm the diagnosis of retinoblastoma, determine the location and extent of the tumor(s), laterality of disease, and complete staging. Photographic documentation in the operating room is recommended.11
Fundus Examination
Retinoblastoma classically presents with single or multiple nodular, white or cream-colored masses often with prominent intralesional blood vessels. There are three primary clinical patterns of retinoblastoma growth: endophytic, exophytic, and diffuse infiltrating (Figure 4). Endophytic growth occurs when the tumor grows from the retina into the vitreous cavity. These tumors break through the internal limiting membrane of the retina and cause vitreous seeding, wherein small pieces of viable tumor break into the vitreous cavity. Clinically, seeding presents as vitreous haze and floating opacities.
Exophytic growth occurs when the tumor expands in the subretinal space (Figure 5). Exophytic retinoblastomas often cause significant exudative retinal detachments that can obscure the tumor and be associated with subretinal seeding. Larger tumors can present a combination of these growth patterns.
The least frequent growth pattern and the most challenging to identify clinically is diffuse infiltrating retinoblastoma. These tumors do not have a discrete mass, rather they infiltrate throughout the retina, causing diffuse thickening without classic calcification, and are often associated with chemosis, pseudohypopyon, and pseudovitritis.
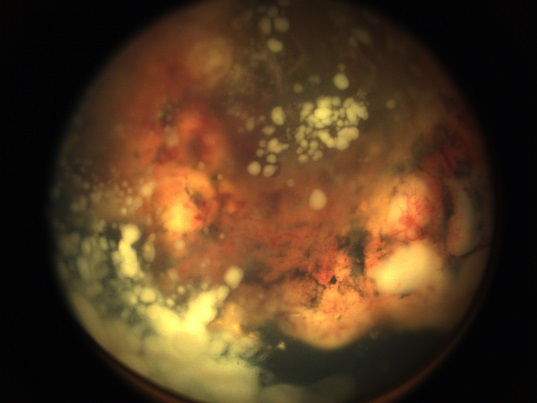
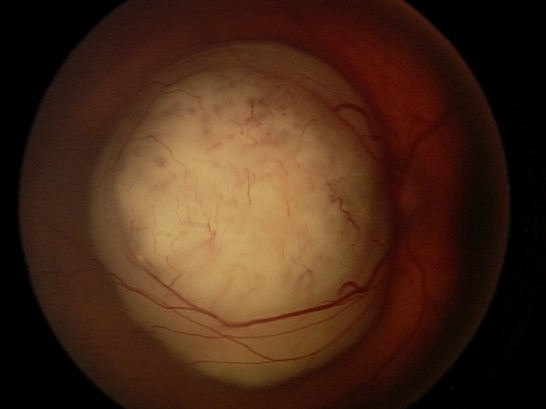
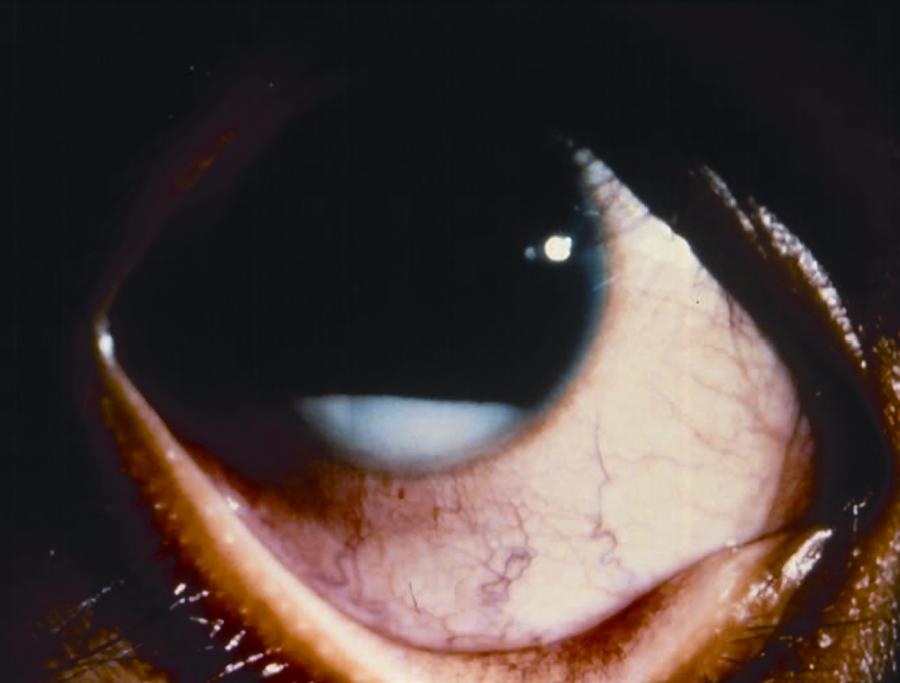
Figure 4. Examples of endophytic (left), mixed endo- and exophytic (right), and pseudohypopyon in a patient with diffuse infiltrating (bottom) retinoblastoma.
Ancillary Diagnostic Tests
Additional testing should include B-scan ultrasonography.11 Ultrasonography will help define tumor height and thickness as well as confirm associated retinal detachment and diffuse intralesional calcification, which is pathognomonic for retinoblastoma.
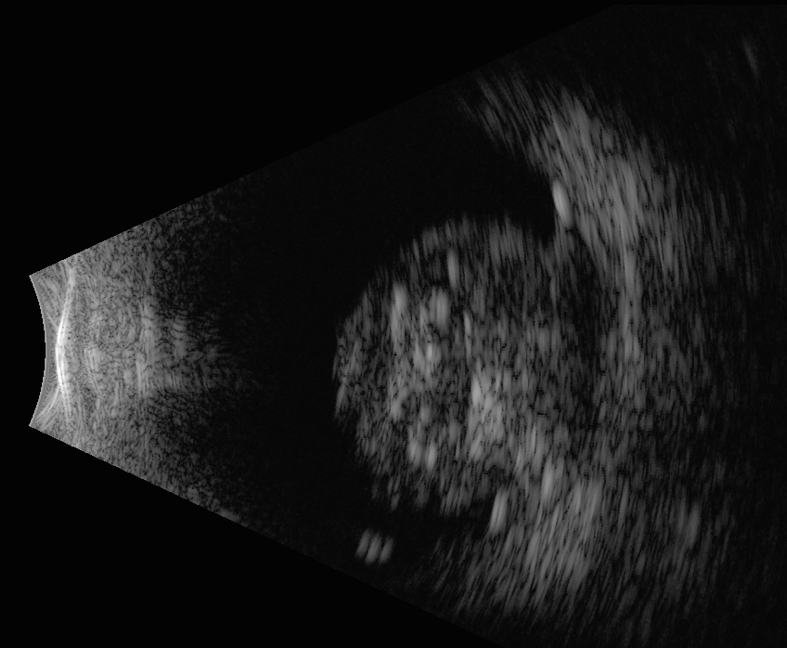
Figure 5. B-scan ultrasound of retinoblastoma. Note the dome-shaped retinal-based mass with intralesional calcification.
Computerized tomography (CT) scan can help demonstrate the presence or absence of calcium deposits and help define the size of the tumor, however, CT is often avoided in young children and even more so in children with a possible genetic cancer syndrome. Magnetic resonance imaging (MRI) is recommended at initial staging to evaluate for optic nerve involvement and extraocular extension and also as screening for concomitant primitive neuroectodermal tumor (trilateral retinoblastoma, Figure 6). Bone-marrow examination or lumbar puncture can be performed in patients if there is concern regarding the extent of disease, particularly with extraocular extension, to rule out cerebrospinal fluid (CSF) or bone-marrow metastases. However, if the disease is confined to the globe, this is not done routinely.
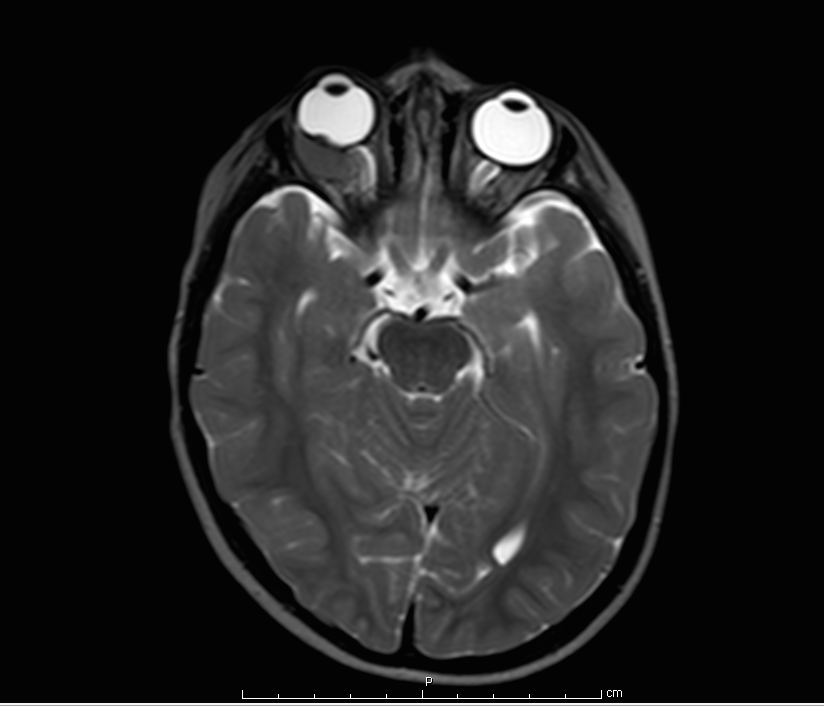
Figure 6. MRI scan showing a right-sided intraconal orbital mass hypointense to the vitreous. Biopsy confirmed this to be retinoblastoma. (Image courtesy of Children's Hospital of Los Angeles Retinoblastoma Service)
Differential Diagnosis
Retinoblastoma is the most critical diagnosis to rule out in the differential for leukocoria; however, loss of the normal red reflex can be caused by other, more benign entities such as a cataract, Coats disease, persistent fetal vasculature (PFV), retinopathy of prematurity, retinal detachment, toxocariasis, choroidal coloboma, vitreous hemorrhage, myelinated retinal nerve fibers, and other retinal tumors, such as astrocytic hamartoma. Corneal opacities can also produce a white reflex, but this can be easily differentiated from leukocoria on clinical exam. Differentiating retinoblastoma from conditions such as Coats disease, PFV, or toxocariasis can be a clinical challenge.
Ocular toxocariasis can cause a white, peripheral retinal mass with a similar appearance to retinoblastoma. Toxocariasis is usually unilateral, and if acute, can be associated with more signs of inflammation such as injection, pain, photophobia, and anterior chamber or vitreous cells than is typically seen with retinoblastoma. A history of fever, eosinophilia, pneumonitis, or hepatosplenomegaly would be suggestive of systemic manifestations of peripheral larva migrans. Serum titers positive for Toxocara canis further support the diagnosis.
PFV is a congenital condition, and leukocoria is noticed very early in life, often at birth. It is typically a unilateral condition, and eyes tend to be microphthalmic. A cataract is often present and can be associated with elongated ciliary processes, which is pathognomonic for PFV. There can also be a stalk, best seen on ultrasonography, between the posterior aspect of the lens and the optic nerve. If present, these features help differentiate PFV from retinoblastoma.
Coats disease presents with abnormally dilated, tortuous vessels associated with significant exudation, which can cause a lipid-laden mass with associated telangiectatic neovascularization. The exudate in Coats disease is more yellow than white because of the presence of cholesterol. Coats disease is typically unilateral and predominantly affects boys between 6 and 8 years of age, which is older than the typical patient with retinoblastoma.
B-scan ultrasonography is helpful in differentiating retinoblastoma from these conditions. The presence of diffuse intralesional calcification associated with a mass favors the diagnosis of retinoblastoma. A noncalcified retrolental mass and a short axial length compared to the contralateral eyes favors a diagnosis of PFV.
Fluorescein angiography (FA) can be helpful to distinguish between retinoblastoma and Coats disease. Both diseases can show dilated and torturous vessels, microaneurysms, intraretinal microvascular anomalies, and areas of retinal nonperfusion. Classically, Coats disease shows focal telangiectasias of small to medium caliber vessels with "light-bulb" aneurysms and profuse leakage into the subretinal space in late phases of the FA. Vessel abnormalities on multiple levels on FA are characteristic of retinoblastoma, particularly in the setting of exophytic tumors. The multiple levels are due to involvement of the retinal vessels overlying the tumor as well as intrinsic tumor vessels. This finding is not seen in Coats disease.13
Management
General treatment
The priorities in the treatment of retinoblastoma are to preserve life, preserve the globe, and preserve vision, in that order. Minimizing side effects and complications of treatment is also of paramount importance in these very young patients. Enucleation remains the definitive treatment of intraocular retinoblastoma, particularly in the majority of patients who present with advanced unilateral disease with poor visual prognosis. Treatment modalities that can be successful in globe salvage include systemic chemotherapy with focal consolidation modalities and intra-arterial chemotherapy. For small tumors, focal consolidation therapy alone can be effective. This includes cryotherapy, laser photocoagulation, hyperthermia, and plaque irradiation.12 External beam radiation therapy (EBRT) is avoided if possible given the associated side effects and should be avoided in children < 12 months of age, but still has a role in the treatment of recurrent tumors and seeding. Intravitreal chemotherapy is a relatively recent addition to the treatment arsenal and in many centers has supplanted EBRT for the treatment of vitreous seeding.
Medical Treatment of Intraocular Retinoblastoma
Chemoreduction
Prior to the 1990s, EBRT played a central role in the treatment of retinoblastoma. Due to disappointingly high recurrence rates, chemotherapy was limited largely to the treatment of metastatic cases. However, with long-term follow-up, clinicians realized how significant an impact EBRT had on the prevalence of secondary tumors, especially in patients with germline mutations. It is estimated that 38.2% of patients with hereditary retinoblastoma will develop a secondary malignancy with an associated long-term mortality rate of 26%.14,15 The risk of second malignancy is increased more than threefold by EBRT, especially if the patient is less than 1 year of age.16 The growing reluctance to use EBRT coincided with the rise of more effective chemotherapeutic regimens with an improved toxicity profile in young children.
Systemic chemotherapy in the setting of retinoblastoma is often termed chemoreduction because it is administered to shrink the tumor and allow subsequent treatment with focal consolidative therapy (Figure 7). Shrinking the tumor increases the success of focal therapies, which are less successful with larger tumors.17 Focal therapy is directly destructive to tumor cells and also breaks down the blood ocular barrier and increases penetration of chemotherapeutic agents into the eye.18 Today, systemic chemotherapy, applied in conjunction with local therapy, is one of the main globe-salvaging options in retinoblastoma management.19
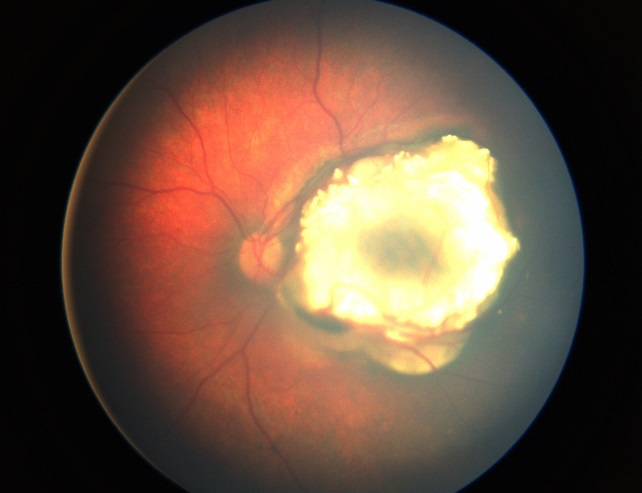
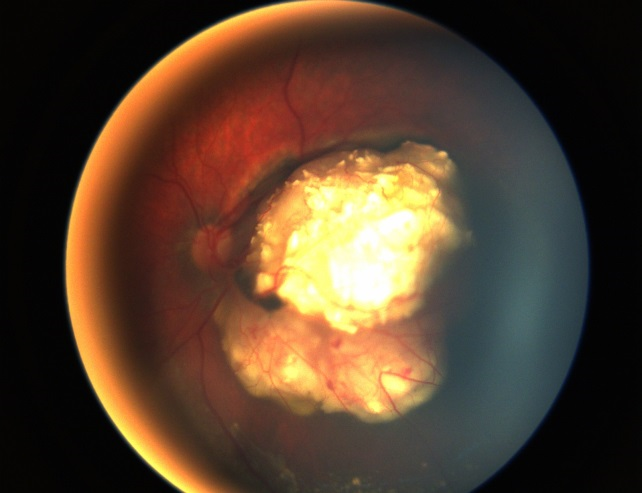
Figure 7. Treated Group B retinoblastoma after systemic chemotherapy and local consolidation (left) with a massive tumor recurrence inferior to the tumor 2 months after the last chemotherapy (right). (Image courtesy of Children's Hospital of Los Angeles Retinoblastoma Service)
The Children's Oncology Group (COG) currently has multiple trials evaluating chemotherapy regimens in conjunction with focal consolidation. Group A eyes are often easily managed with focal therapies alone, therefore no COG trial exists for this group. For Group B tumors, a two-agent protocol involving vincristine and low-dose carboplatin along with local therapy is being evaluated (COG ARET0331). Another trial is evaluating outcomes of Group C/D tumors treated with 3‑drug systemic chemotherapy and sub-Tenon injection of carboplatin (COG ARET0231). Both of these trials have finished recruitment and results are forthcoming. In general, enucleation is recommended for Group E eyes and attempts to salvage these eyes are associated with risk of metastatic disease. There are no current COG trials aimed at treatment of Group E eyes. Children with tumors massively involving the optic nerve, orbit, or brain, or tumors that are present at distant sites are currently being enrolled in a COG Group F trial.
Intra-Arterial Chemotherapy
The quest for local delivery for chemotherapeutics is not new. From intravitreal injection and subconjunctival injection to more complex delivery systems incorporating fibrin sealant, clinicians have been evaluating the possibility for local delivery of antineoplastics around the eye for decades. These therapeutic approaches have the purpose of improving intraocular drug penetration while minimizing systemic toxicity.20
In past years, significant attention has been focused on intra-arterial delivery of chemotherapeutic agents. Based on early attempts in Japan to cannulate the carotid artery directly to deliver antineoplastic drugs to the eye, Abramson and colleagues modified the Japanese protocol to cannulate the ophthalmic artery.18 This technique has been called supraselective intra-arterial chemotherapy. Using melphalan, Abramson and colleagues reported response rates superior to systemic chemoreduction, especially when used as primary versus salvage therapy. However, side effects have also been described including sectoral occlusive choroidopathy, and concerns exist about the potential to cause strokes with this method of delivery.21,22 The total dose of whole-body radiation given with multiple fluoroscopies also remains undefined. A randomized clinical trial has been recommended to determine the efficacy and safety of this approach and the optimum chemotherapeutic agent. The COG is currently enrolling patients with unilateral Group D disease in this trial for treatment with intra-arterial chemotherapy.
Intravitreal Chemotherapy
Tumor control of vitreous seeds remains one of the most challenging aspects of the treatment of retinoblastoma. This is due mainly to their resistance to systemic and intra-arterial chemotherapy. Although the work with intravitreal chemotherapy started in Japan with Kaneko and colleagues, it was Munier and colleagues. who elegantly demonstrated the safety measures necessary to avoid tumor spread into the orbit.23 With this method, no patients had extraocular spread or metastases with a median follow-up of 22 months. The most common and expected toxicity is a localized salt-and-pepper retinopathy restricted to the peripheral retina around the injection site, indicating a higher melphalan concentration at this level and thus further increasing the security against tumor spread.
The specific indication and timing of intravitreal chemotherapy is yet to be defined, but it can become an important second-line therapy for recurrent vitreous seeding.
Surgical Treatment of Intraocular Retinoblastoma
In children whose eyes have a significant tumor burden (Groups D and E) and eyes that progress despite conservative treatments, enucleation should be considered.24 When an eye is enucleated for retinoblastoma, the goal is to remove at least 1 cm of optic nerve to ensure that the cut end of the nerve is free from tumor to reduce both the risk of orbital relapse and the need for adjuvant therapy. Some centers harvest fresh tumor tissue in the operating room for genetic studies and tumor bank storage. Tissue harvesting should be done cautiously so as not to destroy the globe's anatomy or create artifacts that might jeopardize pathologic evaluation. At the time of enucleation, the largest possible orbital implant is placed to encourage normal development of the pediatric orbit. These children will also need fitting for an ocular prosthesis.
Medical Treatment of Extraocular Retinoblastoma
Historically, extraocular retinoblastoma was almost universally fatal. In cases in which the retinoblastoma remained confined to the orbit, there was only a 10% survival rate, and cases of survival with metastatic retinoblastoma were anecdotal.25 Nonetheless, as chemotherapeutic regimens have advanced, the prognosis has significantly improved.
Clinicians are now finding success with neoadjuvant chemotherapy for extraocular retinoblastoma limited to the orbit. This can be followed by surgical debulking and radiation if necessary.26,27 For systemic metastases, especially cases with CNS involvement, aggressive treatment with high-dose chemotherapy (HDC) and autologous stem cell rescue (ASCR) is recommended. HDC involves the administration of high doses of chemotherapeutic agents with the aim to overcome tumor resistance and completely eradicate neoplastic cells. Unfortunately, these lethal doses are also myeloablative, and concurrent ASCR must be performed to allow future reconstitution of the bone marrow. Though toxic, case series of survival after this treatment regimen are being reported.28,29
In 2008 the Children's Oncology Group ARET0321 trial was launched to evaluate intensive multimodal therapy including high-dose systemic chemotherapy using four cycles of induction chemotherapy with vincristine, cisplatin, cyclophosphamide, radiation, and autologous stem-cell rescue for extraocular disease. Though promising, treatment efforts remain focused on early detection and treatment to prevent extraocular disease.30
Trilateral Retinoblastoma
In addition to CNS metastases, children with retinoblastoma can develop a concurrent CNS primitive neuroectodermal tumor (PNET). Trilateral retinoblastoma (Figure 8) is the term often applied to a PNET in the setting of bilateral retinoblastoma. It is a rare neoplasm that occurs in patients with germline retinoblastoma mutations.31
The treatment regimen for patients with PNET is similar to that for patients with CNS metastases, and the prognosis is very poor. As such, early detection and treatment of PNETs is recommended. For this reason, many centers perform routine screening brain MRIs for all children with germline retinoblastoma. It has been noted that there was a decrease in the incidence of PNETs in the chemotherapy era, compared to the radiation era. This is hypothesized to be secondary to either a prophylactic effect due to systemic chemotherapy or because fewer patients are treated with external beam radiation.25,26
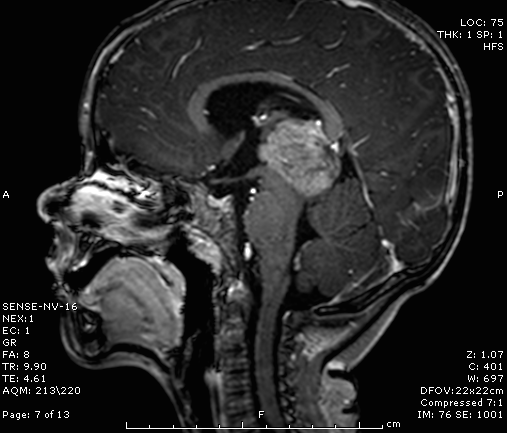
Figure 8. MRI showing an irregular, poorly defined mass consistent with a PNET in a child with bilateral retinoblastoma. (Image courtesy of Children's Hospital of Los Angeles Retinoblastoma Service)
Follow-Up
Children who undergo any form of eye salvaging treatment (external beam radiation, chemoreduction) need frequent follow-up examinations under anesthesia to monitor for recurrence. Tumor regression should be followed closely and the appearance, size, location, and number of tumors documented during each examination. When a tumor regresses after treatment, it can appear as a white, calcific mass or as translucent and similar to fish flesh. A typical schedule would require examinations under general anesthesia every 4–8 weeks until age 3, followed by less frequent examinations if the disease becomes quiescent. Recurrence of retinoblastoma from treated lesions is very common and can occur years after treatment.
Patients with hereditary retinoblastoma also require long-term follow-up with systemic oncologists because such patients have an increased lifetime risk of developing secondary malignancies throughout the body. The most common secondary tumor is osteosarcoma.27 Other tumors include PNETs, fibrosarcoma, and melanoma. Patients who have been treated with radiation are at higher risk for secondary tumors, particularly in the field of treatment. Long-term ophthalmic and oncologic follow-up of all retinoblastoma patients is mandatory, with special vigilance for patients who have germline mutations.
Additional Resources
http://www.eyecancer.com
References
- Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93(1):21-23.
- American Academy of Pediatrics, Section on Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Ophthalmology, American Association of Certified Orthoptists. Red reflex examination in neonates, infants, and children. Pediatrics. 2008;122(6):1401-1404.
- Murphree AL, Benedict WF. Retinoblastoma: clues to human oncogenesis. 1984;223(4640):1028-1033.
- Friend SH, Bernards R, Rogelj S, et al. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323(6089):643-646.
- Abramson DH, Schefler AC. Update on retinoblastoma. Retina. 2004;24(6):828-848.
- Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276-2280.
- Berry JL, Jubran R, Kim JW, et al. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer. 2013;60(4):688-693.
- Chung CY, Medina CA, Aziz HA, Singh AD. Retinoblastoma: evidence for stage-based chemotherapy. Int Ophthalmol Clin. 2015;55(1):63-75.
- Chantada GL, Dunkel IJ, de Dávila MT, Abramson DH. Retinoblastoma patients with high risk ocular pathological features: who needs adjuvant therapy? Br J Ophthalmol. 2004;88(8):1069-1073.
- American Academy of Pediatrics, Section on Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Ophthalmology, and American Association of Certified Orthoptists. Red Reflex Examination in Neonates, Infants, and Children. Pediatrics. 2008;122;1401-1404.
- Lin P, O'Brien JM. Frontiers in the management of retinoblastoma. Am J Ophthalmol. 2009;148(2):192-198.
- Hamel P, Heon E, Gallie BL, Budning AS. Focal therapy in the management of retinoblastoma: when to start and when to stop. J AAPOS. 2000;4(6):334-337.
- Kim JW, Ngai LK, Sadda S, Murakami Y, Lee DK, Murphree AL. Retcam fluorescein angiography findings in eyes with advanced retinoblastoma. Br J Ophthalmol. 2014;98(12):1666-1671.
- Eng C, Li FP, Abramson DH, et al. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85(14):1121-1128.
- Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23(10):2272-2279.
- Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. 1998;105(4):573-579; discussion 579-580.
- Shields JA, Shields CL, De Potter P. Photocoagulation of retinoblastoma. Int Ophthalmol Clin. 1993;33(3):95-99.
- Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. 2008;115(8):1398-1404, 1404 e1391.
- Shields CL, De Potter P, Himelstein BP, Shields JA, Meadows AT, Maris JM. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114(11):1330-1338.
- Abruzzo TA, Geller JI, Kimbrough DA, et al. Adjunctive techniques for optimization of ocular hemodynamics in children undergoing ophthalmic artery infusion chemotherapy. J Neurointerv Surg. 2015;7(10):770-776.
- Abruzzo T, Patino M, Leach J, Rahme R, Geller J. Cerebral vasoconstriction triggered by sympathomimetic drugs during intra-atrerial chemotherapy. Pediatr Neurol. 2013;48(2):139-142.
- Shields CL, Bianciotto CG, Jabbour P, et al. Intra-arterial chemotherapy for retinoblastoma: report No. 2, treatment complications. Arch Ophthalmol. 2011;129(11):1407-1415.
- Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96(8):1078-1083.
- Mourits DL, Hartong DT, Bosscha MI, Kloos RJ, Moll AC. Worldwide enucleation techniques and materials for treatment of retinoblastoma: an international survey. PLoS One. 2015;10(3):e0121292.
- Rootman J, Ellsworth RM, Hofbauer J, Kitchen D. Orbital extension of retinoblastoma: a clinicopathological study. Can J Ophthalmol. 1978;13(2):72-80.
- Chantada G, Fandiño A, Casak S, Manzitti J, Raslawski E, Schvartzman E. Treatment of overt extraocular retinoblastoma. Med Pediatr Oncol. 2003;40(3):158-161.
- Kiratli H, Bilgiç S, Ozerdem U. Management of massive orbital involvement of intraocular retinoblastoma. 1998;105(2):322-326.
- Kremens B, Wieland R, Reinhard H, et al. High-dose chemotherapy with autologous stem cell rescue in children with retinoblastoma. Bone Marrow Transplant. 2003;31(4):281-284.
- Leal-Leal CA, Rivera-Luna R, Flores-Rojo M, Juárez-Echenique JC, Ordaz JC, Amador-Zarco J. Survival in extra-orbital metastatic retinoblastoma:treatment results. Clin Transl Oncol. 2006;8(1):39-44.
- Chantada GL, Doz F, Orjuela M, et al. World disparities in risk definition and management of retinoblastoma: a report from the International Retinoblastoma Staging Working Group. Pediatr Blood Cancer. 2008;50(3):692-694.
- Ibarra MS, O'Brien JM. Is screening for primitive neuroectodermal tumors in patients with unilateral retinoblastoma necessary? J AAPOS. 2000;4(1):54-56.