JUL 20, 2016
Cataract/Anterior Segment
This prospective study shows that the custom-made, flexible silicone iris prosthesis ArtificialIris (HumanOptics) is an effective therapeutic option for traumatic iris defects, with good functional and aesthetically pleasing outcomes.
Since the FDA rejected the Morcher black capsular tension ring device, options are limited for patients with congenital aniridia or traumatic iris loss. These patients can suffer great psychological strain, often with severe glare sensitivity and cosmetic disturbances. Should the FDA approve this iris prosthesis, patients would have a new treatment option beyond iris print contact lenses, sunglasses or simple iris prostheses. An article in the July 2016 issue of Eyenet Magazine discusses the advances and challenges of iris implants.
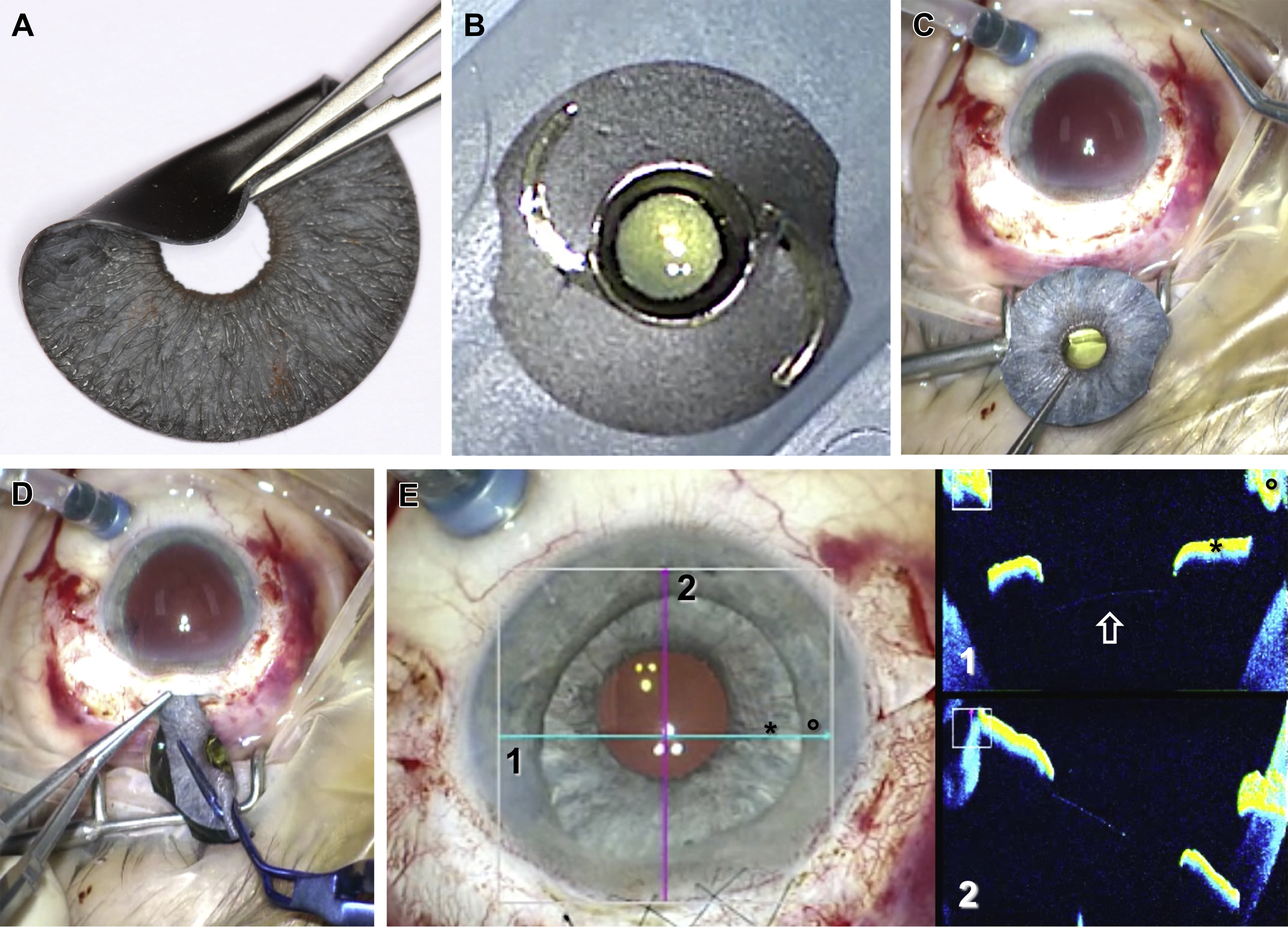
Each custom prosthesis is handcrafted so that its color matches the fellow eye. The pupil size is fixed at 3.35 mm and features an undulated edge that replicates the pupillary frill of a natural iris. The implant is cut to size using a trephine and inserted into the ciliary sulcus using a sclerocorneal approach. It is available with and without embedded polymer fiber meshwork, the earlier being stiffer and allows the surgeon to suture the iris prosthesis.
This is the first study detailing outcomes with the ArtificialIris in 32 patients (32 eyes). The authors found that BCVA and IOP were not significantly different after implantation. Contrast sensitivity using the Pelli-Robson chart was significantly increased (P=0.014) and endothelial cell count was significantly decreased (P=0.003). Complications were noted in 4 patients, with 2 experiencing dislocation or subluxation of the implant.
Patients reported significant improvement in glare and cosmetic disturbance (both P<0.001). Overall mean patient satisfaction was high at 8.91 on a 10-point scale. Every patient said they would undergo the treatment again.