Three elderly women are now legally blind after receiving an unapproved 'stem cell' treatment for AMD.
The procedures were performed in the summer of 2015 at a for-profit clinic in Florida called Bioheart Inc., since renamed US Stem Cell.
Ophthalmologists from Bascom Palmer Eye Institute who later treated the women detailed their horrific complications in a report published this week in the New England Journal of Medicine. The report is accompanied with a blistering editorial disparaging the regulatory loopholes that allow such clinics to operate.
In June 2016, the Academy addressed the issue of unlicensed clinics in a statement calling on the FDA to tighten regulations and increase investigations into treatments given outside of clinical trials.
All 3 women received a slurry of cells extracted from fat tissue liposuctioned from their abdomen. The ‘stem cells’ were agitated in cell-wash solution, given a vague enzyme treatment and centrifuged. The pelleted cells were mixed with platelet-dense plasma and then immediately injected into both eyes simultaneously.
Within a week, the women, aged 72 to 88, presented with pain and significant vision loss caused by retinal detachment, vitreous hemorrhage and lens dislocation. Ajay E. Kuriyan, MD, shared a video demonstrating how he and his colleagues managed treatment in one of the women.
Prior to the treatment, the women had experienced a loss of vision from their disease, yet all were still able to drive. One year after the treatment, their vision ranged from legally blind (20/200) to complete lack of light perception.
Thomas A. Albini, MD, who treated 2 of the 3 patients at Bascom Palmer, described the procedure as “off-the-charts dangerous”.
"That's what led to these horrible results,” said Dr. Albini. “Nobody practicing good medicine would ever do an experimental procedure on a patient on both eyes on the same day.”
When Dr. Albini examined the women just 2 to 3 days after the injections, vision had reduced to counting fingers and hand motion. The patients went on to develop severe proliferative vitreoretinopathy and profound zonular weakness. The authors hypothesize some of the damage was caused by the toxic effects of cell preparation enzymes, such as trypsin, contaminating the injection.
All 3 patients sought the injection after finding a listing on ClinicalTrials.gov for an approved research trial on adipose derived stromal stem cells for dry AMD, sponsored by and located at Bioheart. The women paid $5,000 each, and later reported they believed they were participating in the study. However, according to the official listing, the trial was withdrawn in September 2015, shortly after it was posted and prior to any official enrollment.
“The provision of autologous cellular ‘therapies’ outside the experimental clinical trial setting — and on a for-profit basis — is a gross violation of professional and possibly legal standards,” said George Q. Daley, MD, PhD, a stem cell researcher and dean of Harvard Medical School, who penned the editorial. “Patients have to be wary and tell the difference between the snake oil salesmen who are going to exploit them and the kind of slow, painstaking legitimate clinical trials that are also going on."
According to US Stem Cell’s scientific director Kristen Comella, the proposed study design, including the plan to inject both eyes, had received institutional review board approval. She also stated that only 3 patients received the treatment, yet they were not part of the trial and she would not confirm they are the same 3 cases reported in the New England Journal of Medicine. Yet Comella was quoted by the New York Times as stating the trial never began because the first 3 cases “ended the way they ended, so we decided not to go forward with any additional patients.”
Clinics such as US Stem Cell have popped up around the country claiming to treat a myriad of diseases, avoiding FDA oversight by using minimally-manipulated autologous cells, rather than induced pluripotent (iPSCs) or embryonic stem cells. However, while the latter 2 have demonstrated promise for AMD treatment in early, sanctioned safety and efficacy studies, the benefits of autologous stem cell therapies remain unproven.
According to an NPR article, the FDA is in the process of finalizing 4 new guidelines aimed at clarifying how stem cells may be used.
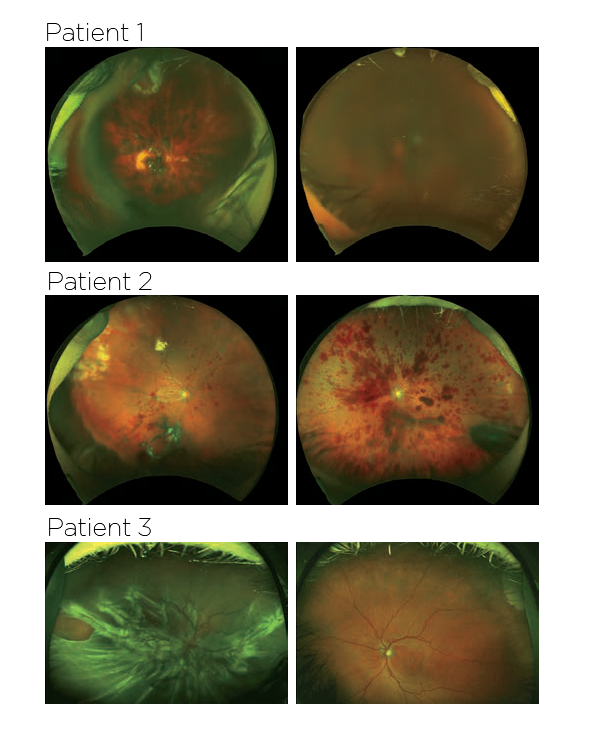
Images taken from the New England Journal of Medicine, showing the fundus photographs from the 3 patients.