Modified monovision or "mini-monovision," which requires a smaller interocular diopteric power difference between eyes than traditional monovision, appears to be a safe and effective treatment for presbyopia. With the use of aspheric monofocal equiconvex IOLs to achieve "blended vision," not only are the photopic phenomena associated with multifocal IOLs minimized, but a larger number of patients can be considered as potential candidates.
A smaller difference
With IOL-based monovision, which has demonstrated the ability to correct presbyopia effectively, one eye is corrected for distance and the other for near.1 Historically, surgeons have aimed for 2.0 to 2.5 D of anisometropia. Based on the defocus test we perform in the office,2 I have found that most patients are unable to tolerate a difference this large. Moreover, commonly used items, such as cell phones, tablets and laptops fall in the intermediate zone. Traditional monovision does not provide clear vision for this intermediate range.
I perform the Defocus Test as follows:
- Determine the dominant eye
- Place the distance prescription in the phoropter. Occlude the nondominant eye (near eye)
- Add -0.25D increments to distance eye (dominant eye) while the patient is viewing his/her best line of acuity. Stop adding minus diopters when sustained blur is reported.
- Occlude the distance eye (dominant eye) and add +0.25D increments to the near eye (non-dominant eye) until sustained blur is reported.
- Note the interocular difference (usually at least 1 D).
- Open both eyes with this new starting point and alternately or simultaneously add -0.25D increments to the near eye and +0.25 D increments to the distance eye until sustained blur is reported or the difference is not tolerated.
- Note this greater difference of usually 2D or greater.
- The test results lie between these two endpoints, generally between 1D and 1.25D.
For mini-monovision, I aim for the IOL power difference between the eyes to be no more than 0.75D. I target the dominant eye for emmetropia and the nondominant eye between -0.5D and -0.75D, with power adjustment made to the nondominant eye based on the result of the dominant eye. To date, we've had little trouble with patient adaptation due likely to the small interocular diopteric difference. A greater difference between the eyes may lead to contrast sensitivity, stereopsis and binocular visual acuity issues.
Minding the aberrations
A key factor to take into consideration is the aberration profile of the IOL selected for monovision. In the past, spherical IOLs were used. Now, aspheric IOLs are available ranging from 0 to -0.27 µm of spherical aberration (SA). The negative asphericity of the implant is used to replicate the youthful lens, which neutralizes the positive SA of the cornea. Studies have demonstrated that a small amount of positive SA may aid with an increased depth of focus,3,4allowing a greater range of overall vision.
The IOL I use for my mini-monovision approach, the Softec HD (Lenstec Inc., St. Petersburg, Fla.), has a neutral SA, with the additional advantage of availability in 0.25D increments between 15.0 to 25.0 D. This allows for more precise IOL power titration. According to the package insert, these IOLs are manufactured with a tolerance of ±0.11 D. These two factors may allow for more predictable refractive outcomes.5,6 Other IOLs available with zero aberration optics are the LI61AO and Akreos MI60 made by Bausch & Lomb (Rochester, N.Y.).
Achieving predictable outcomes
Similar to multifocal IOL patient selection, equal attention is required for the proper selection of traditional monovision candidates. A contact lens trial is recommended to determine whether an individual can tolerate the difference between the eyes.
With the mini-monovision approach, patient selection is not as critical. The key is a predictable refractive outcome that matches the intended target. To achieve our intended refractive goals, we optimize the patient's ocular surface prior to biometry, keratometry and topography measurements. We personalize our A-constants and surgically induced astigmatism.
A retrospective chart review was performed for patients in my practice who selected mini-monovision and had been followed for at least one month after their cataract surgery (group 1: 54 patients, 108 eyes) and at least one year (group 2: 16 patients, 32 eyes).
Inclusion criteria included being at least age 40, any race or gender, with visually significant cataracts and a commitment to undergoing bilateral sequential cataract surgery and without any significant ocular pathology. Those with severe dry eye, retinal or macular pathology, or corneal astigmatism of ≥ 1.51D were excluded. Astigmatism between 0.51D and 1.50D was treated with limbal relaxing incisions. UCVA was measured at distance (20 feet with Snellen), intermediate (32 inches with ETDRS) and near (16 inches with ETDRS).
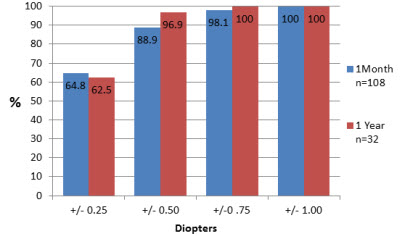
Our results identify that 64.8 percent of eyes followed for at least one month and 62.5 percent of eyes followed for at least one year within 0.25D of the target (Figure 1).
Figure 1: Deviation from intended target
The results showed UCVA to be 20/25 at distance, intermediate and near at rates of 87, 94.4 and 50 percent, respectively, in group 1 (Figure 2) and 93.8, 100 and 50 percent, respectively, in group 2 (Figure 3). Also, 100, 98.2 and 75.9 percent achieved 20/32 UCVA at distance, intermediate and near, respectively, in group 1 and 100, 100 and 87.5 percent in group 2, respectively.
Figure 2: Binocular uncorrected visual acuities (one month postoperative)
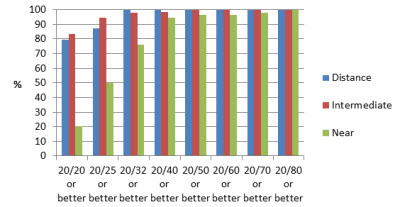
Figure 3: Binocular uncorrected visual acuities (one year postoperative)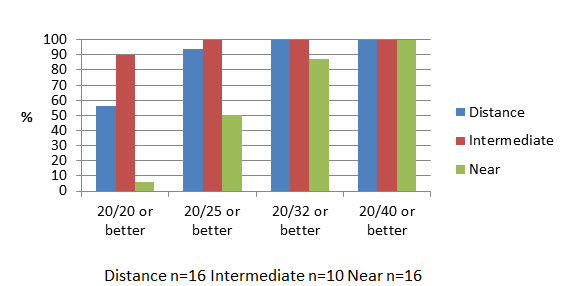
The mean visual acuities for distance, intermediate and near for group 1 were 20/20.1, 20/18.5 and 20/29.5, respectively, and for group 2 were 20/22.8, 20/19.3 and 20/28.6, respectively. Pupil size and postoperative astigmatism were also analyzed. No significant correlation was noted. These results with mini-monovision are comparable to some outcomes utilizing multifocal IOLs.7
Setting realistic expectations
As with all implants, we set expectations prior to surgery by letting patients know that they may need glasses for fine print or for night driving.
Overall, mini-mono IOL implantation offers a safe and effective option for reducing dependency on spectacles after cataract surgery.
References
- Mantry S, Shah S. Surgical management of presbyopia. Cont Lens Anterior Eye. 2004; 27(4): 171-175.
- Maloney, W. Presbyopia success depends on comprehensive preop evaluation. Ocular Surgery News, US Edition, Helio, August 1, 2005.
- Rocha KM, Soriano ES, Chamon W, et al. Spherical aberration and depth of focus in eyes implanted with aspheric and spherical intraocular lenses: a prospective randomized study. Ophthalmology. 2007; 114(11): 2050-2054.
- Yi F, Iskander DR, Collins M. Depth of focus and visual acuity with primary and secondary spherical aberration. Vision Res. 2011; 51(14): 1648-1658.
- Zudans JV, Desai NR, Trattler WB. Comparison of prediction error: labeled versus unlabeled intraocular lens manufacturing tolerance. J Cataract Refract Surg. 2012; 38(3): 394-402.
- Brown DC, Gills JP 3rd, Trattler WB, et al. Prospective multicenter trial assessing effectiveness, refractive predictability and safety of a new aberration free, bi-aspheric intraocular lens. Cont Lens Anterior Eye. 2011; 34(4): 188-192.
- 7. de Vries NE, Nuijts RM. Multifocal intraocular lenses in cataract surgery: literature review of benefits and side effects. J Cataract Refract Surg. 2013; 39(2): 268-78.