Introduction
It has been said “when you see a child suffering from a severe form of vernal keratoconjunctivitis, you instantly feel the dearth in knowledge of its pathogenesis that prevents you from adequately treating the signs and symptoms that will most likely ruin his childhood."1 The term “morning misery” captures the discomfort, blepharospasm, and mucous discharge often manifesting in these patients upon awakening.2
The first description of vernal keratoconjunctivitis (VKC) was by Arlt in 1846 when he reported 3 cases of perilimbal swelling in young patients. Nine years later, Desmarres described the limbal findings now attributed to VKC in “slightly lymphatic children” who were very photophobic. In 1871, von Graefe added “pavement-like granulations of the conjunctiva” to the clinical picture. The association with springtime (vernal means spring) reflects the seasonal increase in signs and symptoms of the condition, particularly the high prevalence in hot, arid environments; affected individuals have disease flares frequently during spring months, but can have signs and symptoms year round.
Clinical Presentation
Demographics
VKC is a disease showing great racial and geographical variation. It is most common and most severe in hot, arid environments such as the Mediterranean basin, West Africa, and the Indian subcontinent.2 In these areas, up to 3% of eye clinic patients present with VKC and 10% of outpatient appointments are made for signs and symptoms related to VKC.3 In Western Europe the prevalence is less than 0.03%. An epidemiologic survey of 6 countries in the European Union (Italy, France, the Netherlands, Norway, Finland, and Sweden) reported that while VKC can be found in both northern and southern Europe, a higher prevalence is in the southern countries (highest in Italy and lowest in Norway).4 Differences in prevalence could be due to the diversity of gene pool, the environment (climate, socioeconomic status, and living styles), and to gene-environment interaction.
The condition is more common in males, although this gender difference is less absolute in tropical climates. The gender discrepancy and the improvement seen during puberty have suggested a role for hormonal influence on disease burden.5 Climate and geography also correlate with the associated concurrence of asthma and eczema. In temperate regions, 45%-75% of patients with VKC have a history of atopy; in contrast, tropical regions have a much lower rate (5%-40%). Limbal VKC is more often seen in patients of African or Asian descent, a racial susceptibility that appears even among those who have migrated to more temperate locales.6
Patients from age 1 to 22 years old (mean 6±3.7 years) may present with signs and symptoms of VKC. Resolution is typically seen between the ages of 8 and 22. The disease usually lasts 4 to 10 years and resolves after puberty.1,3 A family history of allergic diseases occurs in 35.5% of VKC cases, with positivity for other associated allergic states such as asthma, eczema, and/or rhinitis found in 37.1%. A positive skin prick test has been identified in 51.4% of patients.1
Symptoms
Patients with VKC generally present in early to mid-childhood. Symptoms consist predominantly of eye itching, along with tearing, discharge, irritation, redness, blepharospasm, and photophobia.7 The photophobia can be quite severe, with patients frequently presenting in dark sunglasses and hats. Their strong aversion to bright lights renders slit lamp exams challenging, if not altogether impossible.
Signs
Clinically, VKC can be divided into 3 subtypes: conjunctival, limbal, and mixed presentations. Disease severity seems milder in limbal VKC, leading some to suspect that it is the early presentation of a spectrum of disease.8 However, there appears to be variability in the prevalence of certain types, based on geography and atopic history, which suggests that the pathogenesis of the two types may be different.
On external examination, the lids can be erythematous and thickened; a reactive ptosis may be present due to photosensitivity (Fig. 1). The classic finding of giant papillae (> 1 mm diameter) is located most commonly on the upper tarsal conjunctiva. Lid eversion must be performed in order to visualize them (Fig. 2). The tarsal conjunctiva develops a cobblestone appearance and, in active disease, can have mucus accumulation between the papillae. In the limbal form, the conjunctiva may show a fine papillary reaction. Here the predominant findings are gelatinous limbal papillae associated with epithelial infiltrates called Horner-Trantas dots (Fig. 3). These are focal collections of degenerated eosinophils and epithelial cells.
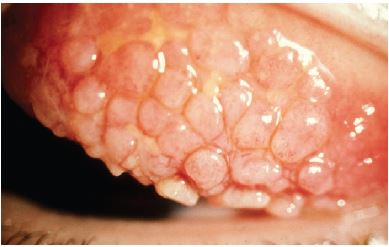
Figure 1. Palpebral vernal keratoconjunctivitis, upper eyelid. Basic and Clinical Science Course, Section 6. Pediatric Ophthalmology and Strabismus.
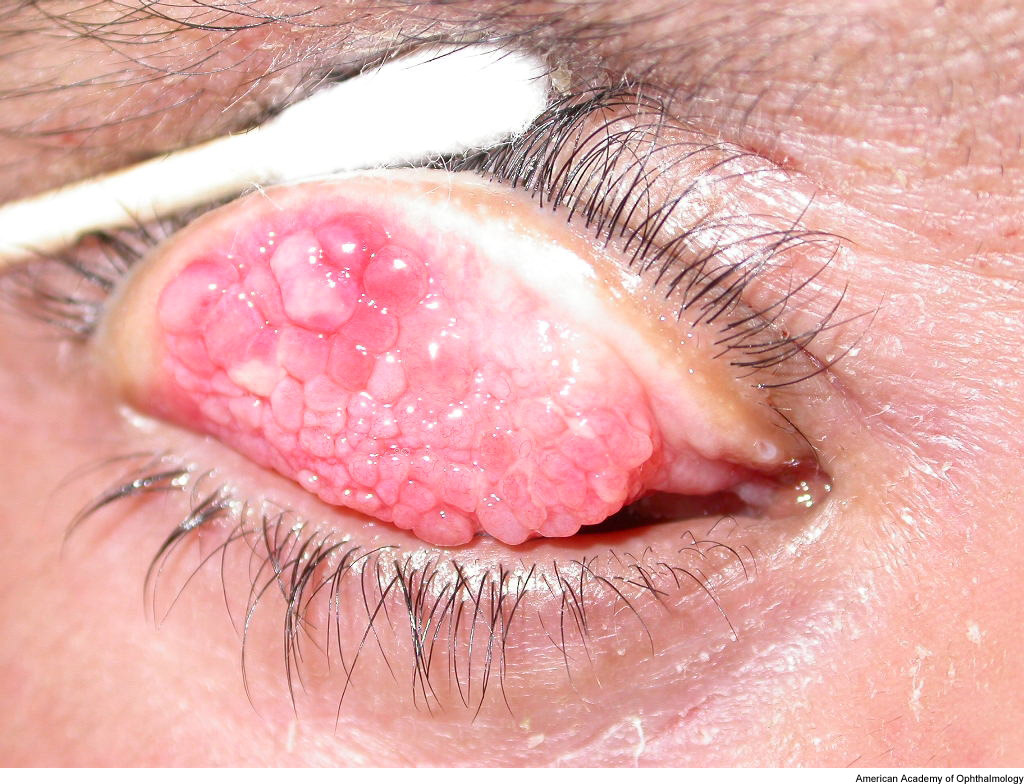
Figure 2. Giant papillae in vernal keratoconjunctivitis. American Academy of Ophthalmology.
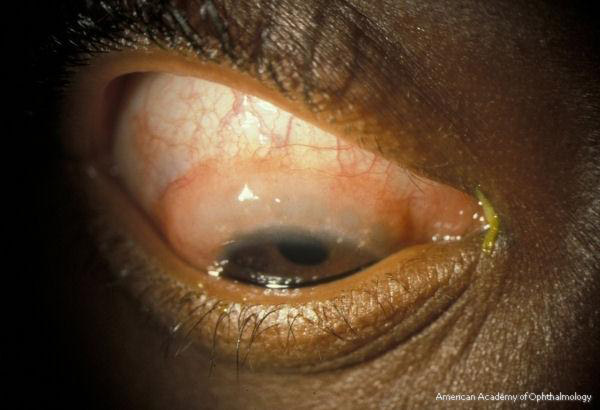
Figure 3. Horner-Trantas dots in vernal keratoconjunctivitis. American Academy of Ophthalmology.
The cornea may become involved in VKC, and the corneal changes range from mild (punctate epithelial erosions) to severe (macroerosions and ulcers). Active palpebral disease may lead to development of superficial corneal neovascularization. Macroerosions form when inciting agents (eg, eosinophilic major basic protein) released from the epithelium of the upper tarsal conjunctiva progress to corneal epithelial necrosis.9 Epithelial erosions may heal completely, with excellent visual outcomes; however, in severe or neglected cases, mucus and calcium deposition can prevent re-epithelialization, and a shield ulcer develops (Fig. 4). Waxing and waning gray-white lipid deposits in the peripheral, superficial stroma create an arcuate infiltrate known as pseudogerontoxon.2
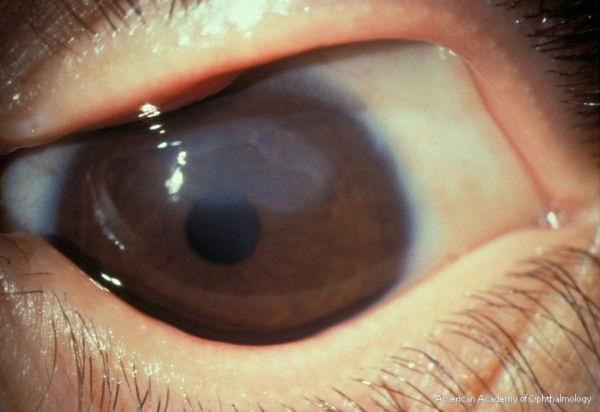
Figure 4. Shield ulcer. American Academy of Ophthalmology.
Other conditions may coexist in children with VKC. Higher incidences of keratoconus (potentially due to eye rubbing) and abnormal corneal topography patterns have been reported.10 Complications of chronic steroid use, including steroid-induced cataracts and glaucoma, have been described in up to 20% of patients.11
Pathophysiology
VKC has been designated in the newest classification of ocular surface hypersensitivity disorders as both an IgE- and non-Ig E-mediated ocular allergic disease.12 Support for the role of IgE-mast cell activation in disease burden comes from findings such as IgE in serum, tear cytology, increased number of mast cells in conjunctival tissue, clinical observations of allergen exposure and symptom exacerbation, and association with other atopic states (Fig. 5).1,7 However, not all VKC patients have positive skin tests and disease flairs do not always follow environmental allergen burden.

Figure 5. Tear cytology of a VKC patient: epithelial cell fragment with numerous adherent eosinophils (May Grunvald, 400 Â ) [Reprinted from Progress in Retinal and Eye Research, volume 21, issue 3, Andrea Leonardi, Vernal keratoconjunctivitis: pathogenesis and treatment, pp. 319-339, May 2002, with permission from Elsevier.]
As expected, increased numbers of CD4+ Th2 lymphocytes are expressed in the conjunctiva of patients with VKC; these cells drive IgE-mediated type I hypersensitivity reactions. Th2-generated cytokines and interleukins promote the synthesis of IgE. This environment promotes mast cell degranulation, histamine release, and the recruitment of other inflammatory cells upon exposure to an allergen. Overexpression of pro-inflammatory cytokines, chemokines, growth factors, and enzymes are found in VKC. Eosinophils and eosinophil-derived major basic protein (MBP) and cationic protein, neurotoxins, and collagenases, in particular matrix metallopeptidase (MMP)-9, have been shown to damage the corneal epithelium and the basement membrane, causing corneal involvement in VKC.13 This environment of chronic inflammation creates tissue remodeling and the clinically observed signs of keratoconjunctivitis.
Treatment
The management of VKC lends itself to a stepwise sequence based on disease severity (Table 1). Symptoms of itching, burning, and irritation can be managed with cool compresses and saline rinses. Preservative-free artificial tears can be used liberally. These simple measures are reported by patients to provide significant symptomatic relief.
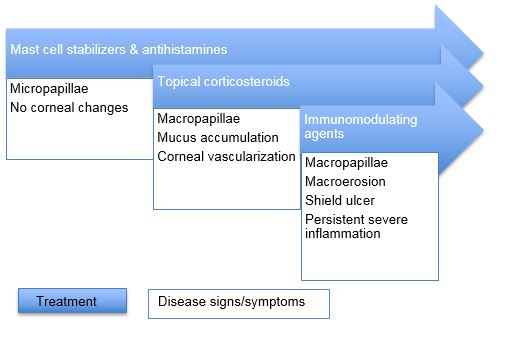
Table 1. Stepladder for treatment of VKC based on disease severity
Among topical medications, antihistamines (levocabastine 0.05%, emedastine 0.05%) and mast cell stabilizers (sodium cromoglycate 2%-4%, lodoxamide 0.1%) appear to provide some comfort. Interestingly, despite the major role of histamine in this disease, antihistamines alone have not proven overwhelmingly successful. For instance, topical levocabastine was found to be inferior to lodoxamide in alleviating ocular symptoms and signs such as itching, tearing, and photophobia.14 Dual-acting agents with activity against H1 receptors and mast cell degranulation (olopatadine 0.1%) have been very successful in the treatment of allergic conjunctivitis and are used in cases of VKC.
Topical acetylcysteine 5%-10% has been used to reduce mucus adherence to the cornea during exacerbations. Other agents that have been tried with varying degrees of efficacy in a limited number of studies include mitomycin-C and ketorolac.15,16
Corticosteroids are very effective, particularly in quieting disease exacerbations. However, their side-effect profile (cataract, glaucoma, herpetic infection) renders them an unpopular selection for long-term management. Use of antihistamine/mast cell stabilizers as maintenance therapy and then pulse therapy with topical steroids during disease exacerbations is a common practice. Prednisolone, fluorometholone, and dexamethasone have been selected for this purpose. With adequate monitoring, this is acceptable. However, loteprednol – an agent with less of an effect on intraocular pressure – has been found to be just as effective as prednisolone in VKC.17
With their ability to inhibit T-cell activation, interest in the efficacy of cyclosporine A and tacrolimus has emerged. Significant improvement has been reported using 2% formulations of cyclosporine, response was rapid but did reoccur after cessation of the drop. Use is safe in children and adverse reactions are primarily limited to stinging with installation.18 Lower strength formulations (cyclosporine 0.05%) appear to be effective, although more expensive.19 Use of topical tacrolimus has shown promise, with studies reporting impressive and rapid reduction in the size of the giant papillae. It also appeared to be more easily tolerated than cyclosporine in its side-effect profile.18
It is rare that VKC patients require a surgical approach. Surgical removal of corneal plaque is recommended only in severe, persistent cases to allow corneal re-epithelialization. Giant papillae excision with intraoperative mitomycin-C may be considered with severe mechanical ptosis and large, coarse papillae with corneal changes.1
Prognosis
The natural history of VKC is one of resolution, specifically with the arrival of puberty. Nonetheless, there is a relative lack of information on the long-term history of the disease. In one study with a median follow-up period of 47 months, only 29.8% of patients had complete recovery, some improvement in symptoms was seen in 35.4%, 31.7% reported no clinical change, and 2.7% had worsening symptoms.20 However, further follow-up may not be available because of the high probability for disease resolution with time.
VKC is a disease with myriad presentations, some of which can generate long-term sequelae. Symptoms can be quite bothersome, and untreated disease can be rather severe; therefore, appropriate treatment can be life- and sight-changing.
References
- Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002: 21: 319–339.
- Buckley RJ. Vernal keratoconjunctivitis. Int Ophthalmol Clin. 1988; 28(4): 303-308.
- O’Shea JG. A survey of vernal keratoconjunctivitis and other eosinophil-mediated external eye diseases amongst Palestinians. Ophthalmic Epidemiol. 2000; 7: 149-157.
- Bremond-Gignac D, Donadieu J, Leonardi A, et al. Prevalence of vernal keratoconjunctivitis: a rare disease? Br J Ophthalmol. 2008; 92:1097-1102.
- Bonini S, Lambiase A, Schiavone M, Centofanti M, Palma LA, Bonini S. Estrogen and progesterone receptors in vernal keratoconjunctivitis. Ophthalmology. 1995; 102:1374–1379.
- Montan PG, Ekström K, Hedlin G, van Hage-Hamsten M, Hjern A, Herrmann B. Vernal keratoconjunctivitis in a Stockholm ophthalmic centre—epidemiological, functional, and immunologic investigations. Acta Ophthalmol Scand. 1999; 77:559-563.
- Vichyanond P, Pacharn P, Pleyer U, Leonardi A. Vernal keratoconjunctivitis: A severe allergic eye disease with remodeling changes. Pediatr Allergy Immunol. 2014; 25:314–322.
- Leonardi A, Busca F, Motterle L, et al. Case series of 406 vernal keratoconjunctivitis patients: a demographic and epidemiological study. Acta Ophthalmol Scand. 2006; 84:406–410.
- Trocmé SD, Hallberg CK, Gill KS, Gleich GJ, Tyring SK, Brysk MM. Effects of eosinophil granule proteins on human corneal epithelial cell viability and morphology. Invest Ophthalmol Vis Sci. 1997; 38:593-599.
- Totan Y, Hepşen IF, Cekiç O, Gündüz A, Aydin E. Incidence of keratoconus in subjects with vernal keratoconjunctivitis: a videokeratographic study. 2001; 108:824–827.
- Tabbara KF. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. 1999; 34(2):88-92.
- Leonardi A, Bogacka E, Fauquert JL, et al. Ocular allergy: recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012; 67:1327–1337.
- Leonardi A, Brun P, Abatangelo G, Piebani M, Secchi AG. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003; 44:3052–3058.
- Verin P, Allewaert R, Joyaux JC, et al. Comparison of lodoxamide 0.1% ophthalmic solution and levocabastine 0.05% ophthalmic suspension in vernal keratoconjunctivitis. Eur J Ophthalmol. 2001; 11:120–125.
- Akpek EK, Hasiripi H, Christen WG, Kalayci D. A randomized trial of low-dose, topical mitomycin-C in the treatment of severe vernal keratoconjunctivitis. Ophthalmology. 2000; 107:263–269.
- Sharma A, Gupta R, Ram J, Gupta A. Topical ketorolac 0.5% solution for the treatment of vernal keratoconjunctivitis. Indian J Ophthalmol. 1997; 45:177–180.
- Oner V, Türkcü FM, Taş M, Alakuş MF, Işcan Y. Topical loteprednol etabonate 0.5% for treatment of vernal keratoconjunctivitis: efficacy and safety. Jpn J Ophthalmol. 2012; 56:312–318.
- Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013; 13(3):308-314.
- Çoban-Karataş M, Özkale Y, Altan-Yaycıoğlu R, et al. Efficacy of topical 0.05% cyclosporine treatment in children with severe vernal keratoconjunctivitis. Turk J Pediatr. 2014; 56(4):410-417.
- Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. 2000; 107:1157–1163.