Cataract/Anterior Segment, Comprehensive Ophthalmology, Retina/Vitreous
A joint task force formed by the American Society of Cataract and Refractive Surgery (ASCRS) and the American Society of Retina Specialists (ASRS) has now identified 23 patients (36 eyes) with hemorrhagic occlusive retinal vasculitis (HORV), a rare and devastating condition linked to the use of intraocular vancomycin.
Based on their findings, investigators provide updated clinical characteristics and recommendations for prevention and management of HORV.
Members of the task force include Dr. David F. Chang, who discusses his perspective on HORV in this interview from AAO 2016, and Dr. Andre J. Witkin, who published the initial series of HORV patients. Dr. Witkin fully describes the clinical features HORV in this video from ASRS 2016.
Of the 36 eyes with confirmed diagnosis included in this report, 11 eyes (6 patients) were presented previously in the July 2015 issue of Ophthalmology, and 3 eyes of 3 additional patients were described in recent case reports.
All patients presented with HORV 1 to 21 days (mean 8 days) after surgery or intravitreal injection. All eyes received vancomycin during procedure via intracameral bolus (33/36), intravitreal injection (1/36), or through the irrigation bottle (2/36).
Visual outcomes were poor overall: 22 eyes (61%) were 20/200 or worse, and 8 eyes (22%) were NLP. Therapy with additional intravitreal vancomycin associated with especially poor outcomes—among 7 eyes that received an additional bolus for treatment of presumed bacterial endophthalmitis, 5 had NLP, 1 had 20/400, and 1 had 20/800. Neovascular glaucoma was the most common secondary complication, occurring in 56% of eyes.
Due to the rarity of HORV, the task force is not recommending that surgeons discontinue use of intraocular vancomycin prophylaxis. However, because all of the cases in this series were associated with vancomycin, they caution surgeons to avoid it in specific cases and consider cefuroxime and moxifloxacin as alternatives.
If you identify a patient with HORV, you can submit the clinical data to the HORV registry at https://www.surveymonkey.com/r/HORV. Patient and surgeon names will be kept confidential.
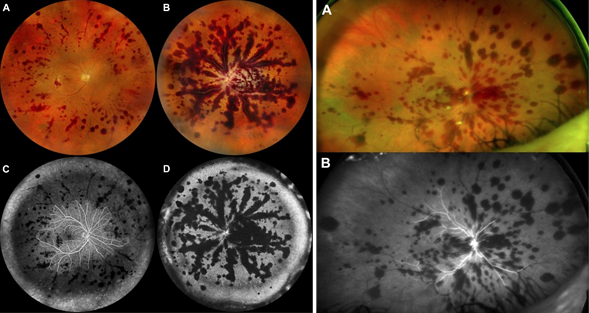
Widefield color and fluorescein angiography of patient 17 (left panel) and patient 21 (right panel) demonstrating diffuse peripheral retinal vascular occlusion, and associated large patches of retinal hemorrhage following the retinal venules.
Clinical characteristics of HORV
- Occurs after intraocular procedure with normal undilated examination on postoperative day
- Delayed onset of sudden painless decreased vision (may be asymptomatic in mild cases)
- Visual acuity often poor at presentation (may be normal in mild cases)
- Mild to moderate anterior chamber and vitreous inflammation, with no hypopyon
- Sectoral intraretinal hemorrhage in areas of nonperfusion (often along venules)
- Peripheral retinal involvement in all cases, with macular ischemia in advanced disease
- Sectoral retinal vasculitis and vascular occlusion on fluorescein angiography, corresponding to areas of hemorrhage
Recommendations for management of HORV
- Avoid intravitreal vancomycin if HORV is suspected.
- Consider an ocular or systemic workup, or both, for other syndromes (e.g., viral retinitis) if the diagnosis is unclear.
- Treat with aggressive systemic and topical corticosteroids; consider periocular or intraocular steroids.
- Consider early anti-VEGF treatment and early panretinal photocoagulation.
Considerations for vancomycin use
- Reconsider using vancomycin with close sequential bilateral cataract surgery, especially if immediate sequential same-day bilateral surgery is performed.
- Be aware that in addition to delayed onset, HORV may not cause symptoms in the first eye, and a dilated retinal examination may be the only way to detect it.
- Consider cefuroxime or moxifloxacin as alternatives.