Introduction
Evaluation of the infant or child with infantile nystagmus syndrome (INS) is very challenging because INS can be an isolated abnormality or appear in association with a wide variety of underlying visual sensory and systemic disorders. Previous studies have shown that approximately 90% of INS occurs in association with a visual sensory disorder of the eye that is either anatomical or functional.1-3 The remaining group of infants has either an underlying metabolic or neurological disorder, a central nervous system (CNS) malformation of the cerebellum and brainstem, hydrocephalus, or an underlying neuroblastoma. The primary physician to whom the family first presents is therefore faced with the daunting task of weeding through a broad spectrum of ophthalmological and systemic disorders. As a result, the infant is usually referred either to an ophthalmologist, to a neurologist, or directly to neuroimaging.
One overlooked aspect of the infant with INS is the potential impact on interpersonal interactions. Eye contact is an important form of interpersonal communication for a nonverbal infant. At least 60% of nonverbal interaction is spent scanning the face.4 When an infant does not fix or follow visual targets, concerns about blindness and a host of comorbidities down the road, especially emotional aspects of well-being, are raised.5 Therefore, as early as possible, it is extremely important to evaluate visual potential, whether good or bad, following a systematic, quantitative approach. Discussions concerning possible surgical interventions are best delayed until the diagnosis is firmly established, relevant ophthalmologic parameters are well-defined, and possible visual benefits, based on objective data, are summarized.
Clinical Assessment
Clinical assessment begins with a careful review of the birth and developmental history. Review of the perinatal history and age at achievement of relevant physical and motor milestones is important. Prenatal exposure to drugs, including illicit drugs and alcohol, may be informative. Family history of nystagmus, albinism, reduced visual acuity, and optic nerve or retinal disease can help narrow the diagnostic possibilities. Likewise, inquiries concerning family members with neurologic, metabolic, or genetic disorders can suggest an underlying systemic disorder.
Comprehensive eye examination begins with an objective assessment of visual acuity, using Teller acuity cards (TACs) in infants or standardized optotype in older children. Infants are presented with a rectangular gray card on which there is a grating embedded on one side that is matched in mean luminance to the gray background (Figure 1). The location of a vertical grating is randomly switched to the left or right of central gaze. As the card is presented to the infant at a standardized distance, the examiner watches for a flick in fixation to the side of the grating through a small hole in the center of the card. To disambiguate a voluntary gaze shift to the pattern grating from a gaze shift generated by the underlying nystagmus, the TACs are held vertically where the gratings are horizontally oriented. Binocular acuity is tested first, and if tolerated, monocular testing is then attempted. Patients with an eccentric or tilted head posture have visual acuity tested in their preferred head position.

Figure 1. Teller acuity cards.
Detection of refractive errors outside the normal range provides additional clues that suggest certain diagnoses. Atkinson and colleagues reported that only 5% of infants 6 to 9 months of age have more than 3 diopters of hyperopia and 0.5% more than 3 diopters of myopia.6 High hyperopia is most consistent with some forms of Leber congenital amaurosis (LCA) or early onset cone–rod dystrophy. Likewise, high myopia is more consistent with albinism, unilateral or bilateral optic nerve hypoplasia (ONH), and blue cone monochromacy (BCM).
Transparency of the cornea, lens, and ocular media is another relevant feature of the ocular examination. The potential impact of opacities of the cornea and lens is based on their bilateral presence, overall size, and severity. Bilateral cataracts are frequently associated with INS in developing countries, owing to late detection and delayed removal. Of note, bilateral congenital cataracts can be associated with macular hypoplasia. Therefore, the presence of INS prior to or following bilateral cataract surgery should prompt evaluation of the macula. Monocular cataracts are associated with gaze-holding instabilities. Birch and colleagues reported a nystagmus incidence of approximately 70% in their cohort of monocular cataracts.7 By comparison, Felius and colleagues reported an incidence of 38% of 83 infants with monocular cataracts who had cataract surgery between 1 and 6 months of age.8 Whether these children have fusion maldevelopment nystagmus, INS, or both is still an open question.
Assessment of pupillary responses is a critical part of the clinical evaluation of the infant with INS. The pupillary light reflex provides two important functions: control of retinal illumination and depth-of-focus. Pupillary area decreases with increasing irradiance over a 9 log unit range.9 The initial constriction of the pupil peaks at 200msec in response to the illuminant; however, the pupillary light response (PLR) persists for 30sec.10-11 The prolonged pupillary light response (PLR) is related to a unique sensitivity to short-wavelength blue light. The observation of a PLR in the absence of rod and cone function,12 or following extinction of the illuminant, suggested there is an additional pupillary pathway. This distinctive light response led to the discovery of melanopsin, a novel photopigment and a subset of intrinsically light-sensitive ganglion cells that express melanopsin.13-14 These cells are referred to as intrinsically photosensitive retinal ganglion cells (ipRGCs). The ipRGCs project to the pretectum (the midbrain region responsible for the PLR), the suprachiasmatic nucleus (SCN) (the area of the brain responsible for circadian rhythms), and the inter-geniculate leaflet. The response of these intrinsically photoreceptive retinal ganglion cells fully accounts for the paradoxical pupillary constriction to extinction of light. The presence of paradoxical pupillary responses has been reported in infants with congenital stationary night blindness and achromatopsia.15-17
Pupillary responses of infants are particularly difficult to evaluate because the pupils are small, owing to delayed development of the dilator muscles and to involuntary constriction to near stimuli. Presentation of the light source in a darkened room at an oblique axis to the direction of gaze helps to distinguish a pupillary response to the illuminant from that of the near response. Documentation of sluggishly reactive or non-reactive pupils bilaterally suggests severe loss of retinal or optic nerve function due to bilateral optic nerve hypoplasia, Leber congenital amaurosis, severe onset cone-rod dystrophy, or congenital retinal dysplasia. Detection of a relative afferent pupillary defect provides objective evidence of better visual potential in the eye that is responsive to light. Of particular note, subtle optic nerve atrophy and hypoplasia are difficult to detect with the indirect ophthalmoscope and are best appreciated through the magnified view of the direct ophthalmoscope. Examination under anesthesia, combined with OCT imaging and ERG testing, may be needed to establish the diagnosis and to assess visual potential.
Reduction of mean acuity in INS with associated visual sensory defect is attributed to the underlying visual sensory defect and exceeds the acuity reduction of isolated INS.18 The rate of acuity development in children with albinism, aniridia, and mild to moderate bilateral optic nerve hypoplasia (BONH) parallels that of normal children. The subset of infants who have severely reduced or no visual orienting behaviors either have delayed visual maturation (DVM) superimposed on a visual sensory disease or severe vision loss due to Leber congenital amaurosis (LCA), cone-rod dystrophy (CORD), congenital stationary night blindness (CSNB), or severe optic nerve hypoplasia or aplasia.19-20 Delayed visual maturation in patients with albinism, and less severe retinal and optic nerve disease is distinguished from INS with severe visual sensory defects by delayed improvements in visual acuity. Infants with INS, reduced visual acuity, and normal fundi are likely to have LCA, cone dysfunction syndrome, or cone-rod dystrophy. Infants with suspected retinal disease need electroretinogram (ERG) testing but, owing to developmental immaturities of the ERG, testing is usually delayed until 6 months to one year of age. It is helpful to routinely coordinate the ERG with an OCT in order to learn more about the anatomical architecture of the retina and to help guide genetic testing.
INS with Visual Sensory Defects
Table 1 provides a list of the obvious visual sensory defects associated with INS, in which an ophthalmological examination adequately reveals the underlying visual sensory defect. The following discussion primarily focuses on those sensory disorders in which the relevant clinical features are more subtle and diagnostic testing provides crucial information. Presumably, the congenital presence or onset of these visual sensory abnormalities before 6 months of age interferes with the development of stable gaze holding, resulting in INS. The physiological basis for stable gaze holding is established early in visual development, presumably initiated by direction selective ganglion cells (dsGC), which represent the major output of the retina.21-24 The retinofugal axons of dsGC corresponding to the fovea and extrafoveal retina then stream within the optic nerves to the lateral geniculate nucleus or to pre-tectal nuclei within the accessory optic system. Functional abnormalities of this shared output likely account for the high prevalence of INS in patients with bilateral congenital corneal opacities, cataracts, macular hypoplasia (albinism and aniridia) and hypoplasia or malformations of the optic nerves. It is postulated that the resulting degradation of visual inputs to the spatiotemporal filters (space-time plots) in striate cortex limits the refinements in downstream cortical and brainstem structures that maintain stable gaze holding.
|
Table 1. Sensory defects associated with INS in which an ophthalmologic exam can reveal the underlying sensory defect.
|
|
INS with obvious visual sensory defect
|
|
A. Bilateral media opacity
|
|
1. Cataract
|
|
2. Cornea
|
|
3. Ocular media (Vitreous)
|
|
B. Bilateral Retinal disease
|
|
1. ROP
|
|
2. Chorioretinal or optic nerve coloboma
|
|
3. Persistent fetal vasculature
|
|
4. Bilateral retinal dysplasia
|
|
5. Norrie disease
|
|
6. Familial exudative retinopathy
|
|
7. Retinoblastoma
|
|
C. Bilateral optic nerve abnormality
|
|
1. Optic nerve aplasia/hypoplasia
|
|
2. Optic nerve coloboma
|
|
3. Optic nerve atrophy
|
|
INS without obvious visual sensory defect
A. Albinism
|
Albinism
Albinism is the sensory disorder most frequently associated with INS.25-26 Albinism includes a group of genetic disorders characterized by a congenital reduction of melanin pigment that can be limited to the eye (ocular albinism) but is more likely to involve the skin, hair, and eyes (oculocutaneous albinism [OCA]) (Table 2). Affected individuals show variable severity of skin and hair hypopigmentation with characteristic eye involvement. Owing to the wide variation in skin and hair pigmentation across racial and ethnic groups, the clinical diagnosis is most consistently confirmed on the basis of the ocular findings. Congenital nystagmus with an abrupt onset during the first 3 months of life is usually the presenting clinical sign. The nystagmus often has a pendular waveform that persists but can evolve into a jerk waveform. Nystagmus severity can be invariant in all gaze positions or can vary with horizontal gaze position. Patients with gaze position differences often adopt a compensatory head turn to align the target at this eccentric gaze position where retinal slip is minimized and visual acuity is optimized. In infancy, the combination of unstable fixation and immature tracking can lead to vision concerns. Although initial visual acuities are below normal, they improve with increasing age, reaching final visual acuities of 20/80 on average. but ranging from 20/25 to 20/200.
The combination of INS and reduced visual acuity are not specific to albinism but are common to many visual sensory disorders. Therefore, hypopigmentation of skin, hair, and eye and specific components of the eye examination provide information that distinguishes albinism. The skin pigmentation may be within the normal range but subnormal relative to that of other family members. Therefore, the skin pigmentation of other family members should be assessed directly or from photographs. Slit lamp examination of the iris may reveal punctate or confluent defects of the pigmented epithelium. Dilated fundus examination reveals the 2 most important diagnostic features. First, the loss of melanin pigmentation within the RPE allows for direct visualization of the underlying choroidal vessels (Figure 2) Secondly, the macula appears to be underdeveloped, owing to the persistence of the inner retinal layers and to abnormal presence of retinal vessels in the normally avascular zone. The macular luteal pigments composed of carotenoids within the superficial retina are still present, giving the macula an orange-brown coloration relative to the hypopigmented peripheral retina.
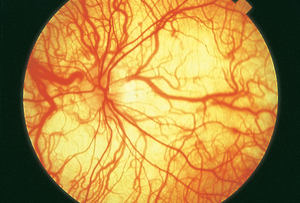
Figure 2. Hypopigmented fundus in albinism. Underlying choroidal vessels can be directly visualized. Basic and Clinical Science Course, Section 12. Retina and Vitreous. American Academy of Ophthalmology. 2016-2017:248. (Courtesy of Carl D. Regillo, MD)
Macular hypoplasia in the context of variable pigment dilution of skin, hair, and eye is the defining hallmark of albinism. Ophthalmoscopic detection of a blunted macular reflex provides subjective evidence of macular hypoplasia. Optical coherence tomography (OCT), which defines retinal anatomy at a resolution near 10 microns, enables semi-quantitation of its severity across patients.27 Therefore, comprehensive assessments of INS patients should include OCT testing using the handheld OCT instrument in younger children and the slit-lamp mounted OCT instrument in older children. In addition, pigmentary dilution of the retinal pigment epithelium predisposes to absent or reduced binocularity and strabismus owing to abnormalities of visual pathway routing. Normally, the ratio of crossed to uncrossed, retinofugal axons is 53:47 in the optic chiasm. In albinism, the ratio of crossed to uncrossed axons is much higher, resulting in the loss of spatial overlap of corresponding nasal and temporal locations in the 2 eyes and the loss of binocular correspondence. The resulting loss of binocularity can be confirmed by demonstrating reduced stereopsis in verbal children or inferred from the presence of strabismus in young children. The preponderance of crossed to uncrossed axons can also be demonstrated by showing asymmetric lateralization of pattern-onset visual evoked potentials (VEPs) under monocular viewing.
|
Table 2. Classification of Albinism
|
|
Clinical condition
|
Molecular defect
|
|
Oculocutaneous albinism type 1(OCA1)
|
|
OCA1A
|
No tyrosinase activity
|
|
OCA1B
|
Reduced tyrosinase activity
|
|
Yellow OCA
|
Reduced tyrosinase activity
|
|
Minimal pigment OCA
|
Reduced tyrosinase activity
|
|
Temperature-sensitive OCA
|
Temperature-sensitive tyrosinase
|
|
Oculocutaneous albinism type 2 (OCA2)
|
|
OCA2
|
P gene mutation
|
|
Brown OCA
|
P gene mutation
|
|
Prader-Willi syndrome
|
Absence of paternally expressed imprinted genes at 15q11.2-q13
|
|
Angelman syndrome
|
Absence of maternally expressed imprinted genes at 15q11.2-q13
|
|
Oculocutaneous albinism type 3 (OCA3)
|
|
OCA3
|
Tyrosinase-related protein 1
|
|
Oculocutaneous albinism type 4
|
Membrane-associated transport protein
|
|
(OCA4)
|
Transporter gene
|
|
Oculocutaneous albinism associated with systemic disease
|
|
Hermansky Pudlak
|
HPS1- HPS8
|
|
Ocular albinism type 1
|
|
OA1
|
G-protein coupled membrane receptor
|
|
OA1 with sensorineural deafness
|
Unknown
|
Macular hypoplasia and INS are not unique to albinism but can appear in the context of aniridia and in bilateral congenital cataracts. Annular deficiency of the central iris, along with corneal pannus, abnormalities of the iridocorneal angle, and propensity to develop glaucoma distinguish aniridia from other forms of macular hypoplasia. Partial deficiency of the central iris may be mistaken for INS until pupillary mydriasis, absence of the pupillary sphincter and central iris structures, and macular hypoplasia are appreciated under the magnification of the slit lamp microscope. Following early removal of bilateral congenital cataracts, the failure to recover normal visual acuity and the persistence of nystagmus should prompt evaluation for macular hypoplasia.
Congenital malformations of the optic nerve
Congenital malformations of the optic nerve bilaterally are frequently associated with INS. Bilateral optic nerve hypoplasia (ONH) is the second most common cause of severe visual impairment with INS in children less than one year of age (retinopathy of prematurity is the first). The clinical hallmarks of bilateral ONH are moderately to severely reduced visual acuity and the presence of unidirectional or multidirectional nystagmus. Visualization of the boundary between the hypoplastic optic nerve and the surrounding retina in the background of an eye in continuous motion is problematic. Options include magnified visualization of the optic nerves with the direct ophthalmoscope or OCT, direct measurement of the optic nerve from fundus photographs obtained with the RETCAM or other instrument, or from magnetic resonance imaging (MRI) of the visual pathways. Asymmetric optic nerve involvement should be distinguished from unilateral disease. The presence of INS indicates there is bilateral involvement, whereas unilateral disease can be associated with fusion maldevelopment nystagmus owing to the loss of binocular input. Borderline ONH is associated with mild macular hypoplasia confirmed by OCT. Therefore, borderline-sized optic nerves with macular hypoplasia can be mistaken for albinism until the reduced nerve fiber layer is appreciated. Another caveat of bilateral ONH is its association with high myopia in which the elongation of the posterior segment magnifies the relative size of the optic nerve. A simple solution is to reduce the apparent size of the optic nerve by the ratio of the age-matched posterior segment length (PSL) and the measured PSL, which can be taken in an examination under anesthesia.
Colobomatous malformations of the optic nerve and/or macula are frequently associated with INS (see http://www.omim.org/ or https://www.genetests.org). It is important to keep in mind that the histologic extent of the malformation extends beyond the boundaries delimited with indirect ophthalmoscopy. Therefore, the presence of INS indicates there is bilateral involvement despite apparent partial or total sparing of the macula. Ocular colobomas can appear as an isolated malformation but usually occur in the context of a genetic or chromosomal abnormality. Therefore, it is important to keep in mind that the nystagmus may be a manifestation of the underlying CNS or vestibular abnormality.
Congenital bilateral optic atrophy
Congenital bilateral optic atrophy is associated with INS. The majority of these infants have already had a neurological examination and neuroimaging studies to exclude mass lesions, hydrocephalus, CNS malformations, and metabolic and white-matter diseases. The normal systemic examination and MRI prompts referral to an ophthalmologist. Detection of optic atrophy should initiate evaluation for hereditary optic atrophy. The most common type is autosomal dominant optic atrophy, type 1 (OPA1). The majority present during the first decade of life, but a subset of patients present in infancy (<10%) without CNS disease and with a normal MRI.28-29 Fundus examination of both parents often reveals subtle optic atrophy in the affected carrier. The diagnosis is confirmed by genetic testing
Additional considerations are Behr optic atrophy and OPA3.30 Behr (1989) described a syndrome of heredo-familial optic atrophy beginning in early childhood that is associated with extrapyramidal tract signs, cerebellar ataxia, mental retardation, urinary incontinence, and pes cavus. Visual acuity stabilized around the 20/200 level. A Behr-like syndrome designated OPA3 or Costeff syndrome was subsequently reported predominantly in females. In addition to optic atrophy, these patients had extrapyramidal movements, particularly chorea, progressive spasticity in half of the patients, and elevated urinary levels of methyl glutaconic acid.31-32 Costeff syndrome was originally attributed to two recessive mutations in the 2-exon gene OPA3.33 A novel third OPA3 gene that encodes 2 transcripts targeted primarily to mitochondria was subsequently identified.34 On the basis of this genetic heterogeneity, this group of diseases is now collectively referred to as 3-Methylglutaconic aciduria types I, II, and III.
Osteopetrosis is a rare cause of optic atrophy with severe progressive visual loss and INS that is easily overlooked. All forms have autosomal recessive inheritance but the clinical severity of disease is highly variable.35 This disorder can result from two different cellular anomalies: a failure to form osteoclasts or a failure to activate mature osteoclasts. The infantile malignant form is due to a mutation in the human GL (grey-lethal) gene and failure to form osteoclasts resulting in early demise within 3-4 weeks of birth.33 Viable forms of osteopetrosis are characterized by increased bone density with narrowing of the internal lumen, resulting in compression of the optic and auditory nerves and pancytopenia. Normal bone thickness is maintained by the regulated balance of bone formation and resorption. Osteopetrosis is characterized by a mutation of an endosomal chloride channel.36 Reduced activity of this chloride channel results in a deficiency of the HCL and proteases needed to resorb bone, resulting in increased skeletal mass. Reduced activity of the chloride channel is also critical to the transmembrane potential of the retinal pigment epithelium, leading to progressive chorioretinal degeneration. A complete blood cell count and femur radiography to evaluate bone density are recommended for infants with optic atrophy and congenital nystagmus for whom there is no obvious basis.
Congenital Hydrocephalus
Hydrocephalus is a common CNS abnormality with frequent onset prenatally or in infancy. Expansion of the ventricles due to increased CSF production or diminished outflow is associated with progressive expansion of the ventricles and compression of the cortical and brainstem parenchyma. At increased risk from pressure damage are the visual radiations, which have an extended anatomic course in the periventricular walls of the lateral ventricles. Dilation of the lateral ventricles, especially the occipital horns, can lead to compressive damage of the visual radiations. In the immature visual system, damage of the visual radiations is associated with optic atrophy and INS likely secondary to trans-synaptic degeneration.37-38 Nonobstructive hydrocephalus and hydrocephalus of the 4th ventricle can be associated with a gaze-evoked nystagmus (GEN) related to pressure effects on the extended neural integrator, which is located in the floor of the 4th ventricle. GEN is distinguished from INS by its minimal amplitude in primary gaze and larger, direction-changing amplitude of the slow phase in lateral gazes.
The recognition of hydrocephalus is often suspected on the basis of clinical findings and then confirmed by neuroimaging evidence of enlarged ventricle(s). However, the diagnosis in which case the clinician needs to look for localizing ophthalmic signs that provide additional supportive evidence can be problematic.39-41 Hydrocephalus is most frequently obstructive (70%) with the site of obstruction localizing to where the CSF outflow is narrowest. These locations include the foramina of Monroe, the posterior 3rd ventricle, the aqueduct of Sylvius, the 4th ventricle, and the 4th ventricle foramina. The remaining 30% of cases are secondary to extraventricular obstruction. CSF in the 4th ventricle normally empties into the cisterna magna and basilar cisterns.42 Obstruction of these cisterns by blood products, inflammatory cells, or tumor can lead to retrograde obstructions of CSF, systemic signs, and distinctive neurologic deficits. Relevant clinical features include history of similarly affected family members, meningitis, intracranial hemorrhage, trauma, or spina bifida. Systemic symptoms and signs include progressive macrocephaly with bulging fontanelles, emesis, lethargy, seizures, focal neurological deficits, and failure to thrive.
Infants with obstructive hydrocephalus at the level of 3rd ventricle and aqueduct of Sylvius have highly characteristic oculomotor findings that reflect the response properties of the closely apposed oculomotor structures of the dorsal midbrain: paralysis of upgaze, light-near dissociation of the pupils, convergence-retraction nystagmus, eyelid retraction (Collier’s sign), and conjugate downgaze (setting sun sign). This constellation of oculomotor deficits is referred to as the dorsal midbrain or Parinaud syndrome. Compression of the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), which is the burst generator for vertical saccades, is associated with paresis of upward saccades and down-gaze position bias.43-45 Of special note, upward smooth pursuits may be intact. Pressure on the vertical gaze integrator (Interstitial Nucleus of Cajal, INC) is associated with vertical gaze-evoked nystagmus.43-45 Pressure on the posterior commissure is correlated with convergence-retraction nystagmus. Additional downstream structures associated with specific clinical findings include pressure on the central caudal nucleus (eyelid retraction or Collier’s sign), the Edinger-Westfall nucleus (light near dissociation) and the Group C vergence neurons in the region of the oculomotor nuclei (convergence retraction, paralysis of convergence or divergence, spasm of convergence, A-pattern XT and downbeat nystagmus.40,46-48
Visual Pathway Tumors
Infants with chiasmal gliomas come to medical attention with clinical features consistent with a diencephalic syndrome and nystagmus.49-52 The nystagmus is frequently monocular, small amplitude (<2deg), high frequency ( >5Hz), and either horizontally or vertically directed. These infants tend to have large tumors having pathological features consistent with diffuse infiltrating glioma ( ). These tumors tend to grow rapidly and to be very aggressive, which may be related to the high frequency of BRAF mutations.53 The nystagmus may be related to the tumor’s close approximation with the accessory optical system (AOS) or asymmetric compression of the retinofugal axons with direction selectivity. Cyclotorsional and rotary nystagmus have been observed associated with compression of the rostral midbrain (INC and midbrain tegmentum), which is consistent with their role in the planning of vertical and cyclovertical eye movements. Collectively, the diencephalic clinical features, early visual loss, optic atrophy, and distinctive nystagmus help to distinguish these infants from those with IN.
Retinal Disorders
Leber Congenital Amaurosis
Leber congenital amaurosis (LCA) is a generic term used to describe a heterogeneous group of retinal dystrophies that are present at birth. Affected infants present in the first few months of life with poor vision and multidirectional nystagmus. The visual impairment is worse in the dark, but a subset can be light sensitive. Visual acuity ranges from 20/40 (Teller acuity card) to no light perception, but most infants present with severely reduced visual orienting behaviors. The fundus can be normal or there can be an atrophic or bull’s eye maculopathy, granular pigmentation of the RPE, superficial white dots, peripheral telangiectasia, chorioretinal atrophy and vascular attenuation. High hyperopia is more prevalent in LCA. The diagnosis is confirmed by ERG testing, which reveals a severely reduced presence or an absence of cone and rod photoreceptor function. Optical coherence tomography (OCT) can reveal 6 distinct layers or a reduced number and poorly defined retinal layers.54
The wide clinical spectrum parallels the genetic heterogeneity with at least 14 different genes associated with LCA to date. Three genotypes (GUCY2D, CEP290 and CRB1) account for 50% of all cases. Each of these genotypes shows a wide range of clinical phenotypes. For example, CEP290 mutations have been documented in patients with LCA, Meckel Gruber, Senior Loken and Bardet Biedl syndromes. Although the onset of disease is in infancy, the retinal degeneration progresses with advancing age. A subset of patients with CRB1 mutations with perivascular sheathing can develop retinal telangiectasis, exudative detachment, and neovascular glaucoma. Spectral domain OCT provides useful information regarding retinal anatomy and the integrity of individual retinal layers. Furthermore, the OCT may provide information about the underlying genotype and progression of disease. For example, CRB1, important for establishment of epithelial polarity, co-localizes with the zonula adherens of the RPE, rod and cone photoreceptors, and Müller glial cells. The youngest child in a family with a CRB1 mutation showed well-delineated retinal layers with preservation of the (OLM), whereas older patients showed coarse lamination and loss of the OLM.
Severe Onset Cone-Rod Dystrophy (SECORD)
Cone–rod dystrophy with onset in infancy is especially problematic because of the regional distribution of cone photoreceptors, phenotypical diversity, and association with a wide range of systemic diseases. Decreased visual acuity and INS are the predominant clinical features of severe onset CORD, owing to the early involvement of cone photoreceptors. The ocular fundi may be normal in appearance or there may be a bull’s-eye or atrophic maculopathy, pigmented retinal stippling or optic nerve pallor. Family history of similarly affected relatives who are otherwise healthy may help to distinguish the isolated genetic cases. Inheritance can be autosomal dominant or recessive and X-linked. Developmental and past medical history is critical for the identification of those with underlying systemic disease. Table 3 provides a list of the systemic diseases that are associated with cone–rod dystrophy.
|
Table 3. Systemic diseases associated with cone-rod dystrophy
|
|
Genetic
|
|
|
|
|
|
|
|
|
|
|
|
Metabolic
|
|
|
|
|
|
|
|
Neurologic
|
|
|
|
|
|
|
|
|
- Primary intra-hepatic cholestasis
|
Achromatopsia
Congenital abnormalities of cone photoreceptor function include a heterogeneous group of genetic disorders. Clinically, these patients present in infancy with photophobia, subnormal visual acuity, conjugate pendular nystagmus, and normal-appearing fundi. Dyschromatopsia is confirmed in older children who fail color discrimination testing (Panel D-15 or Farnsworth Munsell 100 color plates). The presence of dyschromatopsia can only be confirmed in infants in the laboratory setting by showing normal VEP responses to black/white patterns and reduced responses to isoluminant color stimuli. Inheritance is autosomal recessive or X-linked. The typical and most common phenotype is the autosomal recessive form referred to as achromatopsia or rod monochromatism. This disorder has been shown to be due to a genetic mutation in the alpha or beta subunit of the cyclic guanosine monophosphate (c-GMP) gated sodium channel. Confirmation of the diagnosis is by full-field electroretinogram testing which shows normal scotopic responses and severely reduced to extinguished photopic responses.
Congenital Stationary Night-blindness
Congenital stationary night blindness (CSNB) includes a heterogeneous group of genetic disorders of the retina associated with reduced visual acuity ranging from 20/40 to 20/200, diminished vision in the dark, high incidence of nystagmus (50%) and normal fundi. A subset of infants with superimposed delayed visual maturation and lack of visual orienting behaviors can initially be mistakenly thought to have LCA.55 Inheritance is X-linked, autosomal recessive, or autosomal dominant (Table 4). Two forms can be distinguished on the basis of differential ERG responses and dark adaptation testing: complete (cCSNB) and incomplete (icCSNB) stationary night blindness. Complete SNB is characterized by a normal a-wave and a reduced or absent b-wave under scotopic conditions, but a near-normal b-wave in response to a bright flash or a flash flickering at 30 Hz under photopic conditions. In contrast, incomplete SNB shows a reduced b-wave in response to a light flash under scotopic conditions and a near-normal response to a bright flash or a flash flickering at 30 Hz under photopic conditions.
The complete form of CSNB is associated with mutations of the genes GRM6 (metabotropic glutamate receptor 6), NYX (Nyctalopin) and TRPM1 (transient receptor potential cation channel). Each of these mutations is associated with loss of function of rod and cone ON bipolar cells. As a result of the loss of these inputs, the leading edge of the b-wave is squared off, but there is a normal OFF response driven by cone OFF bipolar cells. CSNB1A is caused by mutations of NYX, TRPM1 (transient receptor potential cation channel), and GRM6 encoding metabotropic glutamate receptor 6. The incomplete form of CSNB is associated with mutations of the gene CACNA1F but 2 phenotypes. Affected males have decreased acuity, nystagmus, and myopia. A novel mutation of this gene includes fundus hypopigmentation, foveal hypoplasia, and a protan color defect. This allelic variant is referred to as Aland Island eye disease.
Autosomal dominant
CSNB with abnormal fundi include 2 distinctive entities. One is Oguchi’s disease, in which the fundus has a metallic sheen which diminishes after prolonged dark adaptation. Null mutations of rhodopsin kinase underlie this disease. The second type is Fundus Albipunctatus in which there are multiple yellow-white flecks throughout the retina sparing the macula.
|
Table 4. Inheritance patterns in congenital stationary night blindness
|
|
Inheritance pattern
|
Gene(s) encoded
|
|
X-linked
|
Nyctalopin (NYX)
|
|
Autosomal recessive
|
Metabotropic glutamate receptor 6 (GRM6)
Transient receptor potential cation
Rhodopsin kinase (TRPM1)
|
|
Autosomal dominant
|
Alpha-transducin
|
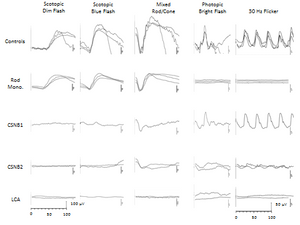
Figure 3. Full-field electroretinogram.
Vestibular disorders and nystagmus
Disorders of the vestibular end organ and the central vestibular pathways are underappreciated causes of nystagmus in childhood. These patients are often misdiagnosed as INS without visual sensory disorder. The hallmark of a unilateral or bilateral peripheral vestibulopathy is a constant velocity, slow phase nystagmus that is elicited in darkness but suppressed in the light. The direction of the slow phase component can be horizontal, torsional, or a combination of both directions when each of three canals is affected. Disease restricted to either the anterior or posterior canal is associated with torsional nystagmus in ipsilateral gaze and vertical nystagmus in contralateral gaze. The nystagmus can be provoked by change in head position or angular rotation of the child around an earth-vertical axis while wearing Fresnel or high hyperopic lenses to blur vision. Another useful clinical test is the rapid head thrust test during which the subject’s head is rapidly turned in alternate directions while the subject is fixating on a stationary, central target. The VOR gain is lower when the head is rotated away from the affected side.
Laboratory confirmation of peripheral vestibular disease includes caloric testing and chair rotation testing. Caloric testing entails irrigation of the external auditory canal with warm/cold air or water to elicit a conjugate eye movement. Inter-aural asymmetries of the induced eye velocity are indicative of peripheral disease that lateralizes to the ear with lower velocity. Chair rotation testing involves sinusoidal rotation of the body with the head fixed around an earth-vertical axis at a peak velocity of 60 deg/s at frequencies ranging from .01 to 0.60 Hz. The gains (peak eye velocity/peak head velocity) for rotation in the clockwise and counterclockwise direction are normally equal. Asymmetrical directional responses between ears, particularly at low rotation frequencies, localize the abnormality to the ear with lower gains. An alternative to chair rotation testing that can be performed in the clinic setting is the head impulse test (HIT). The subject is instructed to fixate on a near target, during which the head is manually rotated clockwise or counterclockwise over short and unpredictable intervals by the examiner. If the visually enhanced VOR gain is low, the subject will generate a corrective saccade to re-align both eyes on the target.
Congenital disorders of the vestibular organ are associated with nystagmus. In the most comprehensive study of the prevalence of vestibular and balance disorders in children, which included data from 561,151 patient encounters, cumulative prevalence of diagnoses related to balance was 0.45% (n=2,546) For 5,793 (1.03%) of patients, the chief complaint was related to balance, and 2,076 (35.84%) also had a vestibular disorder. Balance complaints were recorded for 38% with peripheral disturbances and 21% with central disturbances.56 These percentages probably are low given that many of these children present with dizziness Disorders of the vestibular apparatus, which includes the semicircular canals and the otolithic organ (saccule and utricle), are provoked with specific head movements and can be unilateral or bilateral. Clinical signs of peripheral vestibular disease include an abnormal head posture, episodic nystagmus provoked by head movement, fluctuating visual acuity, unexplained emesis, and delayed motor development. Based on clinical assessment alone, the constant slow phase velocity of vestibular nystagmus is indistinguishable from the exponentially increasing slow-phase velocity of INS. Therefore, the clinical context in which the nystagmus occurs may provide important diagnostic information. The variable presence of chorioretinal colobomas, congenital heart disease, choanal atresia, developmental delay, and ear abnormalities prompts consideration of the CHARGE association. Although the detection of bilateral fundus colobomas can account for the nystagmus, absence of the semicircular canals and dysplasia of the vestibule may underlie a vestibular nystagmus. Congenital infections due to cytomegalovirus (CMV) are associated with hearing loss and vestibular nystagmus owing to viral-mediated damage of the hair cells within the cochlea and vestibular apparatus. A history of bacterial meningitis and/or exposure to systemic antibiotics, particularly aminoglycosides, in the perinatal period is potentially another risk factor for vestibular nystagmus. Aminoglycoside toxicity is characterized by the bilateral loss of hair cells and by nephrotoxicity.
Disorders of central vestibular pathways are characterized by a constant velocity slow phase that may be vertical (upbeat or downbeat), horizontal, cyclotorsional, or a mixed combination of each type. The heterogeneity of the nystagmus waveforms reflects the extensive unilateral and bilateral interconnections of the vestibular nuclei with multiple regions of the brainstem and cerebellum. Central vestibular disorders are poorly suppressed by visual fixation and are not modulated by head movement. Chair rotation testing can show normal, high, low, or asymmetric gains for clockwise and counterclockwise rotation. Localization of vestibular deficits is aided by the assessment of conjugate eye movements because they share neural structures that generate or calibrate slow and fast eye movements. The underlying basis for this shared relationship is that the vestibular nuclei provide the final input to the motor neurons that generate smooth pursuit, and the slow phases of OKN and VOR.
The clinical history and neuroimaging are critical to the evaluation of the infant or child with INS due to an associated disorder of central vestibular pathways. Hypoxic ischemic injury can include damage of the brainstem, including selective damage of the vestibular nuclei.57 Primary hydrocephalus or hydrocephalus secondary to intraventricular hemorrhage is frequently associated with vestibular nystagmus, owing to direct or remote pressure effects on vestibular pathways in the brainstem. Of particular note, the presence of vertical or torsional nystagmus, strabismus, and abnormalities of eye movements may be the more sensitive indicator of increased intracranial pressure (ICP) than CT or MRI (personal observation). A growing number of malformations of the brainstem and cerebellum associated with abnormalities in axonal routing and inter-neuronal connectivity can be associated with vestibular nystagmus as well as other gaze-holding instabilities (GHI).58
|
Table 5. Causes of vestibular-related nystagmus.
|
|
Peripheral
|
- Unilateral peripheral vestibulopathy
|
- Bilateral peripheral vestibulopathy
|
|
|
|
|
|
|
|
|
|
Central
|
|
|
|
|
Joubert Syndrome
Joubert syndrome (JS) is a genetic disorder in which INS (horizontal, pendular nystagmus) is frequently present. A subset of patients may have a seesaw nystagmus characterized by a conjugate horizontal component superimposed on a vertical dysconjugacy. JS is readily distinguished from other forms of INS by the associated clinical findings including developmental delay, hypotonia, ataxia, episodic breathing difficulties in infancy, and eye movement abnormalities. Brain magnetic resonance imaging (MRI) reveals the characteristic molar tooth sign, which refers to the cerebral spinal fluid (CSF)-filled interpeduncular fossa, hypoplasia of the cerebellar vermis, and horizontally oriented and thickened superior cerebellar peduncles.
Clinically the diagnosis of JS should be considered when a developmentally delayed child with hypotonia and nystagmus generates a gaze shift using a head movement rather than an eye movement. This finding, referred to as saccadic initiation failure, reflects the underlying inability to either generate saccades or to generate accurate saccades in a timely manner. This finding should prompt neuroimaging to exclude JS and other forms of oculomotor apraxia. Weiss and colleagues documented oculomotor abnormalities of varying severity in all subjects with JS having eye movement recordings. Half of the subjects failed to generate saccades or generated saccades that showed no consistent relationship with the direction, amplitude or timing of the stimulus. The remaining half generated targeted saccades that were either hypometric or hypermetric. Smooth-pursuit could not be elicited or gains were variably reduced. Horizontal optokinetic nystagmus in response to gratings drifted at velocities of 15, 30, and 45 degrees/s were uniformly reduced. Of note, the oculomotor abnormalities often improve with increasing age. It is important to keep in mind.
Achiasma
Infants with achiasma also come to medical attention because of seesaw nystagmus and reduced visual acuity. Achiasma is a rare and frequently overlooked disorder characterized by failure of the nasal retinofugal fibers to decussate at the optic chiasm.1–6 The nasal and temporal retinofugal fibers of each eye are connected to the ipsilateral visual cortex. The MRI correlate is the lack of a defined optic chiasm, which is normally generated by the anatomical interconnection of both optic nerves. Functional evidence of the diagnosis includes monocular visual fields and visual evoked potentials, both of which are normal in spatial extent, indicating that the nasal and temporal representations of each eye are connected to ipsilateral visual cortex.4,6,9 Functional magnetic resonance imaging (fMRI) confirms that monocular stimulation selectively activates the ipsilateral visual cortex.
Paradoxically, visual acuity is reduced despite normal OCT imaging of the macula, normal cone density amplitude of the central hexagon (2° subtense) of the multifocal ERG, and normal cortical representation of the macula. Furthermore, Weiss and colleagues documented that smooth pursuit, saccadic, optokinetic, and vestibulo-ocular reflex eye movements were conjugate and scaled with the direction and magnitude of the target step, target velocity, or chair rotational velocity. Taken together, these data suggested that visual acuity was limited by eye velocity. As proof of concept, the authors performed a tenotomy and re-attachment of the lateral recti of each eye in an 8-year-old child. After surgery, this child immediately showed reduced eye velocities but improvements in visual acuity were documented one year later (Unpublished data).
Metabolic Disorders
Cobalamin deficiency
Metabolic disorders are a rare but important cause of INS because of the presence of significant co-morbidities and treatment options. Disorders of cobalamins are rare, but mandatory newborn screening has led to increased and earlier detection. Humans have two B12-dependent enzymes; methionine synthase, which methylates homocysteine to form methionine, and methylmalonic acid mutase, which converts methylmalonyl-CoA to succinyl-CoA. In addition, there are many “handlers” that modify dietary B12 and deliver it to its target enzymes. Early onset disease is characterized by hypotonia, developmental delay microcephaly, seizures, and MRI abnormalities (hydrocephalus, white matter edema and attenuation, and progressive cerebral atrophy). Additional findings include glomerulopathy, hemolytic uremia syndrome, and megaloblastic anemia and other pancytopenias. The diagnosis is initially suspected on the basis of elevated levels of homocysteine and decreased levels of methionine. Ocular manifestations are related to CNS disease (cortical visual impairment) and/or progressive retinal degeneration. The presence of nystagmus is highly correlated with reduced retinal function, even when the retina appears to be normal. Ophthalmoscopic examination may reveal an atrophic maculopathy, pigmentary abnormalities, or a normal-appearing fundus. Electroretinograms (ERGs) are performed to quantify retinal function and to follow the progression of the disease and response to treatment. ERG abnormalities are characterized by delay of the a-wave and blunting of the b-wave. Treatment includes hydroxycobalamin (B12), betaine (betaine homocysteine transferase), folic acid, and dietary protein restriction.
|
Table 6. Metabolic causes of nystagmus.
|
|
Metabolic
|
|
|
- Carbohydrate Glycoprotein
|
|
|
|
|
|
|
|
|
Peroxisomal Biogenesis Disorders (PBD)
Peroxisomes are membrane-bound organelles that catalyze the biosynthesis of plasmalogens and bile acids, and α- and β-oxidation of long-chain fatty acids and related compounds. Peroxisomal proteins are encoded by the nuclear genome, synthesized by free polyribosomes in the cytosol, and then bound to receptor molecules of peroxisomes, which internalize them. Peroxisomal disorders are divided into two groups: peroxisomal biogenesis disorders (PBDs) in which there is a generalized deficiency of peroxisomal enzymes deficiency (Zellweger syndrome) or of a single peroxisomal enzyme (like ALD protein in X-linked adrenal leukodystrophy or phytanoyl CoA hydroxylase in Refsum disease). PBDs share the following clinical characteristics: dysmorphic facies. Typically seen are a large fontanel, shallow orbits, broad nasal bridge, anteverted nostrils, psychomotor retardation, hypotonia, hearing loss, and retinal degeneration. Zellweger is the most severe phenotype with severe hypotonia, neonatal seizures, neuronal migration defects, and hepatomegaly. Brain MRI scans feature profound hypo-myelination, small and abnormal gyri, and neuronal heterotopia. Infantile phytanic acid storage disease and infantile Refsum disease are milder forms of Zellweger syndrome. These disorders are characterized by early onset, mental retardation, minor facial dysmorphism, retinitis pigmentosa, sensorineural hearing deficit, hepatomegaly, osteoporosis, failure to thrive, and hypocholesterolemia with potential life expectancy to second or third decade.
Pelizaeus Merzbacher
Pelizaeus Merzbacher disease (PMD) is the most common form of hypomyelinating leukodystrophy (OMIM 312080) with X-linked inheritance. PMD gene encodes two proteins: proteolipid protein (PLP) and DM 20. PLP is expressed by oligodendroglia cells and constitutes the predominant protein in CNS myelin. The predominant clinical findings are infantile nystagmus and cerebellar dysfunction (hypotonia and motor delays). Early onset of nystagmus may lead to a misdiagnosis of INS unless the evaluation includes a developmental assessment and careful examination for optic atrophy. Optic atrophy with paradoxical pupillary responses has been reported.59 Longitudinal evaluations disclose psychomotor regression, and brain MRI demonstrates absence or attenuation and progressive loss of CNS myelin. Molecular testing reveals duplication or missense, insertional or deletional mutations of the PLP gene.
Carbohydrate Glycoprotein Deficiency
The carbohydrate-deficient glycoprotein syndromes include a heterogeneous group of multisystem genetic disorders characterized by defective addition of oligosaccharides to the asparagine moiety of glycoproteins. These N-linked glycoconjugates are an essential moiety of various serum transport proteins (apolipoprotein B, transferrin), hormones (thyroid-stimulating hormone), lysosomal enzymes, and circulating proteins (immunoglobulin G). The large number of potentially defective proteins predisposes affected individuals to multisystem disease with multiple phenotypes. Based on the pattern of isoelectric focusing of transferrin, patients are diagnosed with CDG-1 or CDG-2.
Affected infants present with failure to thrive, feeding difficulties, psychomotor retardation, hypotonia, esotropia, inverted nipples, lipodystrophy, pericardial effusion, and hepatic dysfunction. Liver biopsy reveals steatosis. CT and MRI scan confirm cerebellar hypoplasia. Stark and colleagues reported a 10-month-old with intermittent high-frequency horizontal oscillations superimposed on an underlying conjugate, pendular nystagmus.60
References
- Cogan DG. Congenital nystagmus. Can J Ophthalmol. 1967;2(1):4-10.
- Gelbart SS, Hoyt CS. Congenital nystagmus: a clinical perspective in infancy. Graefes Arch Clin Exp Ophthalmol. 1988;226(2):178-180.
- Weiss AH, Biersdorf WR. Visual sensory disorders in congenital nystagmus. Ophthalmology. 1989;96(4):517-523.
- Berberat J, Jaggi GP, Wang FM, Remonda L, Killer HE. Changes in the amygdala produced by viewing strabismic eyes. Ophthalmology. 2013;120(10):2125-2129.
- Pilling RF, Thompson JR, Gottlob I. Social and visual function in nystagmus. Br J Ophthalmol. 2005;89:1278-1281.
- Atkinson J, Braddick OJ, Durden K, Watson PG, Atkinson S. Screening for refractive errors in 6-9 month old infants by photorefraction. Br J Ophthalmol. 1984;68(2):105-112.
- Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996;37:1532–1538.
- Felius J, Busettini C, Lynn MJ, Hartmann EE, Lambert SR; Infant Aphakia Treatment Study Group. Nystagmus and related fixation instabilities following extraction of unilateral infantile cataract in the Infant Aphakia Treatment Study (IATS). Invest Ophthalmol Vis Sci. 2014;55(8):5332-5337.
- Loewenfeld IE. The Pupil: Anatomy, Physiology and Clinical Applications. Ames: Iowa State University Press, 1993, vol. 1.
- Pong M, Fuchs AF. Characteristics of the pupillary light reflex in the macaque monkey: metrics. J Neurophysiol. 2000;84(2):953-963.
- Gamlin PD, Zhang H, Clarke RJ. Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Exp Brain Res. 1995;106(1):169-176.
- Zaidi FH, Hull JT, Peirson SN, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17(24):2122-2128.
- Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749-754.
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47(7):946-954.
- Barricks ME, Flynn JT, Kushner BJ. Paradoxical pupillary responses in congenital stationary night blindness. Arch Ophthalmol. 1977 Oct;95(10):1800-1804.
- Flynn JT, Kazarian E, Barricks M. Paradoxical pupil in congenital achromatopsia. Int Ophthalmol. 1981;3(2):91-96.
- Price MJ, Thompson HS, Judisch GF, Corbett JJ. Pupillary constriction to darkness. Br J Ophthalmol. 1985;69(3):205-211.
- Weiss AH, Kelly JP. Acuity development in infantile nystagmus. Invest Ophthalmol Vis Sci. 2007;48(9):4093-4099.
- Russell-Eggitt I, Harris CM, Kriss A. Delayed visual maturation: an update. Dev Med Child Neurol. 1998;40(2):130-136.
- Weiss AH, Kelly JP, Phillips JO. The infant who is visually unresponsive on a cortical basis. Ophthalmology. 2001;108(11):2076-2087.
- Yamagata M, Sanes JR. Expanding the Ig superfamily code for laminar specificity in retina: expression and role of contactins. J Neurosci. 2012;32:14402–14414.
- Dhande OS and Huberman AD. Retinal ganglion cell maps in the brain: implications for visual processing. Curr Opin Neurobiol. 2014; 24(1):133-142.
- Cruz-Martin A, El-Danaf RN, Osakada F, et al. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature. 2014;507(7492):358-361.
- Sanes JR, Masland RH. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci. 2015;38:221-246.
- King RA, Hearing VJ, Creel DJ, et al. Albinism. In: Scriver CR, Sly WS, Beaudet AL, eds. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill Companies; 2012.
- Weiss A. Ocular abnormalities in childhood metabolic disorders. In: Nelson LB, Olitsky SE, eds. Harley’s Pediatric Ophthalmology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
- McAllister JT, Dubis AM, Tait DM, et al. Arrested development: high-resolution imaging of foveal morphology in albinism. Vision Res. 2010; 50:810-817.
- Smith DP. Diagnostic criteria in dominantly inherited juvenile optic atrophy: a report of three new families. Am J Optom Physiol Opt. 1972; 49: 183–200.
- Hoyt CS. Autosomal dominant optic atrophy: a spectrum of disability. Ophthalmology. 1980; 87: 245–251.
- Behr C. Die komplizierte, hereditär-familiäre Optikusatrophie des Kindesalters: ein bisher nicht beschriebener Symptomkompleks. Klinische Monatsblätter für Augenheilkunde. 1909: 47: 138–60.
- Sheffer RN, Zlotogora J, Elpeleg ON, Raz J, Ben-Ezra D. Behr’s syndrome and 3-methylglutaconic aciduria. Am J Ophthalmol. 1992; 114: 494–497.
- Costeff H, Elpeleg O, Apter N, Divry P, Gadoth N. 3-Methylglutaconic aciduria in ”optic atrophy plus.” Ann Neurol. 1993; 33: 103–104.
- Anikster Y, Kleta R, Shaag A, Gahl WA, Elpeleg O. Type III 3-methylglutaconic aciduria (optic atrophy plus syndrome, or Costeff optic atrophy syndrome): identification of the OPA3 gene and its founder mutation in Iraqi Jews. Am J Hum Genet. 2001; 69: 1218–1224.
- Huizing M, Dorward H, Ly L, et al. OPA3, mutated in 3-methylglutaconic aciduria type III, encodes two transcripts targeted primarily to mitochondria. Mol Genet Metab. 2010; 100(2):149-154.
- Siatkowski RM, Vilar NF, Sternau L, Coin CG. Blindness from bad bones. Surv Ophthalmol. 1999;43(6):487-490.
- Van Wesenbeeck L, Odgren PR, Coxon FP, et al. Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J Clin Invest. 2007;117(4):919-930.
- Gills JP Jr, Wadsworth JA. Degeneration of the inner nuclear layer of the retina following lesions of the optic nerve. Trans Am Ophthalmol Soc. 1966;64:66-88.
- Lujan BJ, Horton JC. Microcysts in the inner nuclear layer from optic atrophy are caused by retrograde trans-synaptic degeneration combined with vitreous traction on the retinal surface. Brain. 2013;136(Pt 11).
- Katz DM, Trobe JD, Muraszko KM, Dauser RC. Shunt failure without ventriculomegaly proclaimed by ophthalmic findings. J Neurosurg. 1994;81:721–725.
- Nguyen TN, Polomeno RC, Farmer JP, Montes JL. Ophthalmic complications of slit-ventricle syndrome in children. Ophthalmology. 2002;109(3):520-524; discussion 524-525.
- Aring E, Andersson S, Hård AL, et al. Strabismus, binocular functions and ocular motility in children with hydrocephalus. Strabismus. 2007;15(2):79-88.
- Barkovich JA, Raybaud CR. Pediatric Neuroimaging. 5th ed. Philadelphia: Lippincott Williams and Wilkins/Wolters Kluwer. 2012.
- Pasik P, Pasik T, Bender MB. The pretectal syndrome in monkeys. I. Disturbances of gaze and body posture. Brain. 1969;92(3):521-534
- Pasik T, Pasik P, Bender MB. The pretectal syndrome in monkeys. II. Spontaneous and induced nystagmus, and "lightning" eye movements. Brain. 1969;92(4):871-884.
- Leigh JR, Zee DS. The Neurology of Eye Movements. 5th ed. New York: Oxford University Press. 2015.
- Cobbs WH, Schatz NJ, Savino PJ. Midbrain eye signs in hydrocephalus. Trans Am Neurol Assoc. 1978;103:130.
- Corbett JJ. Neuro-ophthalmologic complications of hydrocephalus and shunting procedures. Semin Neurol. 1986;6:111–123.
- Tzekov C, Cherninkova S, Gudeva T. Neuroophthalmological symptoms in children treated for internal hydrocephalus. Pediatr Neurosurg. 1991;17:317–320.
- Schulman JA, Shults WT, Jones JM Jr. Monocular vertical nystagmus as an initial sign of chiasmal glioma. Am J Ophthalmol. 1979;87(1):87-90.
- Farmer J, Hoyt CS. Monocular nystagmus in infancy and early childhood. Am J Ophthalmol. 1984;98(4):504-509.
- Good WV, Koch TS, Jan JE. Monocular nystagmus caused by unilateral anterior visual-pathway disease. Dev Med Child Neurol. 1993;35(12):1106-1110.
- Toledano H, Muhsinoglu O, Luckman J, Goldenberg-Cohen N, Michowiz S. Acquired nystagmus as the initial presenting sign of chiasmal glioma in young children. Eur J Paediatr Neurol. 2015;19(6):694-700.
- Ho CY, Mobley BC, Gordish-Dressman H, et al. A clinicopathologic study of diencephalic pediatric low-grade gliomas with BRAF V600 mutation. Acta Neuropathol. 2015;130(4):575-585.
- Pasadhika S, Fishman GA, Stone EM, et al. Differential Macular Morphology in Patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-Related Leber Congenital Amaurosis. Invest. Ophthalmol. Vis. Sci. 2010; 51(5):2608-2614. doi: 10.1167/iovs.09-3734.
- Weleber RG, Tongue AC. Congenital stationary night blindness presenting as Leber’s congenital amaurosis. Arch Ophthalmol. 1987; 105:360-365.
- O’Reilly RC, Morlet T, Nicholas BD, et al. Prevalence of vestibular and balance disorders in children. Otol Neurotol. 2010;31:1441-1444
- Volpe JJ, Inder TE, Darras BT. Neurology of the Newborn. 5th ed. Philadelphia: Saunders Elsevier, 2008.
- Barkovich AJ, Millen KJ, Dobyns WB. A developmental and genetic classification for midbrain-hindbrain malformations. Brain. 2009;132:3199-3230.
- Koeppen AH, Ronda NA, Greenfield EA, Hans MB. Defective biosynthesis of proteolipid protein in Pelizaeus-Merzbacher disease. Ann Neurol. 1987;21(2):159-170. paradoxic pupil 3827224.
- Stark KL, Gibson JB, Hertle RW, Brodsky MC. Ocular motor signs in an infant with carbohydrate-deficient glycoprotein syndrome type Ia. Am J Ophthalmol. 2000;130(4):533-535.