Micro-invasive Glaucoma Surgery and the Traditional Paradigm
The management of childhood glaucoma differs from that of adult-onset glaucoma. Instead of offering surgery after conservative therapies have proven inadequate, surgery is the first-line treatment in many cases of childhood glaucoma. Traditionally, if angle surgery (goniotomy and/or trabeculotomy) can be performed, it has been the first choice of procedures. If angle surgery cannot be performed or has failed, a glaucoma drainage device (GDD) or filters are often needed for pressure control.1,2 Transscleral cyclophotocoagulation is sometimes offered when the intraocular pressure (IOP) remains uncontrolled despite tube shunt implantation.3
Over time, the IOP-lowering effect of all glaucoma surgeries decreases, while the cumulative risk of complications increases. This is especially true in children with glaucoma, because they have the expectation of living with the disease longer than those with adult-onset glaucoma. Because multiple surgeries are often required over a child’s lifetime, preserving conjunctival tissue during the initial surgery may increase the likelihood of success in subsequent surgeries. Several large, prospective trials in adult patients suggest that most late postoperative complications (eg, persistent corneal edema, persistent motility disturbance, tube erosion, tube obstruction) are associated with either hardware implantation or the presence of an anterior filtering bleb (eg, bleb leak, hypotony maculopathy, endophthalmitis).4 Thus, it stands to reason that bleb-less and hardware-free procedures may be particularly suitable for children, especially if such procedures can be performed without incising conjunctiva. As of 2019, there are no randomized clinical trial data available on the safety and efficacy of glaucoma surgery in children.
In 2012, Saheb and Ahmed defined the 5 cardinal features of micro-invasive glaucoma surgery (MIGS): 1) ab interno microincisional approach; 2) minimally traumatic to the target tissue; 3) at least modestly efficacious; 4) extremely high safety profile, and 5) rapid recovery with minimal impact on the patient’s quality of life.5 Since then, the number of MIGS procedures and devices has multiplied (Table 1). MIGS procedures do not violate conjunctiva, though many still result in filtering blebs and/or require implantation of hardware. Some have proposed the alternative description of “micro-incisional” (instead of “micro-invasive”) when describing this group of procedures, highlighting that the procedures’ ab interno approach may involve significantly more intraocular manipulation and increase the risk of iatrogenic corneal or lenticular injuries.6 For the various newer MIGS devices to have a role in the routine treatment of pediatric glaucoma, they ought to outperform the goniotomy knife (and its variations), with the additional cost justified by the added benefit. In general, factors that affect the choice of surgical procedures include angle status (open/unincised, open/incised, or closed) and corneal clarity. As of 2019, there is no high-quality outcomes data available for children and infants treated with MIGS procedures.
Table 1. Commercially available micro-invasive glaucoma devices and procedures as of June 2018.
|
Surgical options
|
Bleb-forming
|
Requires hardware implant
|
Theoretical advantages over goniotomy
|
Image
(Click to enlarge)
|
|
Ab interno canaloplasty (iTrack, Visco360, Omni720**)
|
|
|
May augment goniotomy by dilating the Schlemm canal; no theoretical benefit over goniotomy if performed without incision in the trabecular meshwork
|
Figure 1. Omni720 Viscosurgical System |
|
CyPass suprachoroidal micro-stent**
|
|
x
|
Bypasses episcleral outflow apparatus, may offer an alternative to tube shunt or filter after angle surgery failure*
|
|
|
Endoscopic cyclophotocoagulation
|
|
|
Can be used in angle closure
|
Figure 2. Intra-procedural photograph of endoscopic cyclophotocoagulation
|
|
Goniotomy
|
|
|
|
Figure 3. Goniotomy
|
|
Goniosynechialysis
|
|
|
Treats angle closure
|
Figure 4. Goniosynechialysis
|
|
Kahook dual blade**
|
|
|
Excises trabecular tissue, may decrease risk of cicatricial closure of trabecular cleft
|
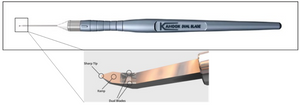
Figure 5. Kahook Dual Blade
|
|
iStent**
|
|
x
|
None
|

Figure 6. iStent
|
|
iStent Supra**
|
|
x
|
Bypasses episcleral outflow apparatus, may offer an alternative to tube shunt or filter after angle surgery failure
|
Figure 7. iStent Supra
|
|
Trabectome**
|
|
|
Electroablation of trabecular tissue, may decrease risk of cicatricial closure of trabecular cleft
|

Figure 8. Trabectome surgical package
|
|
Gonioscopy-assisted transluminal trabeculotomy (iTrack, blunted prolene, Visco360, Trab360, Omni720)**
|
|
|
Incises circumferentially the trabecular tissue through one main incision
|
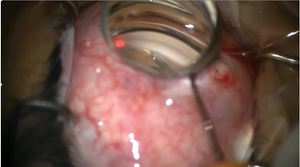
Figure 9. Gonioscopy-assisted transluminal trabeculotomy
|
|
Xen gel stent**
|
x
|
x
|
Bypasses episcleral outflow apparatus, may offer an alternative to tube shunt or filter after angle surgery failure
|
Figure 10. Xen gel stent
|
*Voluntary global recall in effect as of September 2018.
**iTrack (Ellex iScience, Inc. Fremont, CA, USA), Visco360, Trab360 and Omni720 (Sight Sciences, Inc. Menlo Park, CA, USA), Cypass suprachoroidal microstent (Alcon, Inc., Fort Worth, TX, USA), Kahook dual blade (New World Medical, Rancho Cucamonga, CA, USA), iStent (Glaukos, San Clemente, CA, USA), Trabectome (NeoMedix Corporation, Tustin, CA, USA), Xen gel stent (Allergan, Dublin, Ireland).
Angle Open, Cornea Clear
Although currently there is no high-quality evidence that supports the use of MIGS in the management of childhood glaucoma, many of the innovations offer theoretical advantages over the traditional surgical approach. Based on the MIGS criteria, one can argue that goniotomy, a main staple of childhood glaucoma surgery since the 1940s, is the original MIGS, given its simplicity, safety, and efficacy.7 To perform goniotomy, an intraocular blade is introduced through a clear corneal incision and advanced across the anterior chamber to the opposite angle. Under direct gonioscopic visualization, the trabecular meshwork is incised. Approximately 120° to 180° of angle can be treated through a single clear corneal incision, and the procedure can be repeated in the same or a subsequent session through different corneal incisions to maximize the extent of angle treatment. Short-term success after single or multiple goniotomies (defined as IOP less than 21 mm Hg) ranges from approximately 50% to 60% in glaucoma following cataract surgery8 and glaucoma associated with uveitis, to approximately 85% in primary congenital glaucoma.9,10 Goniotomy is contraindicated when the cornea is not sufficiently clear to allow visualization of the target tissue.
Several gonio-ablative instruments, such as the electrosurgical trabeculectomy device (Trabectome; NeoMedix Corporation, Tustin, CA, USA) and the dual-blade trabeculectomy device (Kahook dual blade; New World Medical, Rancho Cucamonga, CA, USA), enhance the traditional goniotomy approach by ablating or excising—instead of merely incising—the trabecular meshwork and the inner wall of the Schlemm canal, which theoretically decreases the risk of postoperative cicatricial closure of the trabecular cleft. Like goniotomy, both the electrosurgical trabeculectomy device and the dual-blade trabeculectomy device are introduced through a clear corneal incision and advanced across the anterior chamber to the opposite angle. Under direct gonioscopic visualization, the device engages the trabecular meshwork. The electrosurgical trabeculectomy device electroablates the trabecular meshwork tissue to expose the trabecular cleft, while the dual-blade device simultaneously incises the anterior and posterior trabecular meshwork, such that a trabecular ribbon is freed and removed to expose the trabecular cleft. Neither device allows circumferential treatment of the anterior chamber angle through a single corneal incision and both require direct visualization of the angle. These devices have the theoretical advantage over goniotomy of decreasing the risk of cicatricial closure of the trabecular cleft. Like goniotomy, they are contraindicated when the cornea is not sufficiently clear to allow visualization of the target tissue.
Gonioscopy-assisted transluminal trabeculotomy (GATT) is an approach that permits ab interno circumferential angle incision without violating the conjunctiva or creating a scleral flap.11,12 In the classic description of this procedure, a main and an auxiliary clear corneal incision are created approximately 90 degrees apart. Following a traditional goniotomy through the main incision, a filament (either a blunted polypropylene suture or an illuminated fiberoptic microcatheter) is introduced through the auxiliary corneal incision and directed through the goniotomy into the Schlemm canal, using intraocular microforceps. The filament is advanced until the Schlemm canal is cannulated circumferentially, and the 2 ends of the filament are then pulled to rupture the inner wall of the Schlemm canal in a purse-string fashion, which results in a circumferential incision of the trabecular meshwork. A modified GATT procedure can be performed using an ab interno filament trabeculotome (Trab360, Visco360, or Omni720; Sight Sciences, Inc. Menlo Park, CA, USA), which can incise the anterior chamber angle in a pseudocircumferential fashion through a single corneal incision. After the creation of a traditional goniotomy, the filament trabeculotome is advanced across the anterior chamber to the opposite angle, and the retractable filament is deployed into and cannulates approximately 6 clock hours of the Schlemm canal. When the device is withdrawn from the eye, the filament incises the cannulated portion of the Schlemm canal, creating a 180° trabeculotomy. The procedure is then repeated through the same corneal incision and goniotomy opening in the opposite direction, thus creating a pseudo-circumferential ab interno trabeculotomy. While both approaches have the theoretical advantage over goniotomy by treating the angle circumferentially, the filament trabeculotome treats the entire angle through a single corneal incision, requires significantly less intraocular manipulation than a classic GATT procedure, and may confer a lower risk of iatrogenic injury to intraocular structures.13 Like goniotomy, these procedures are contraindicated when the cornea is not sufficiently clear to allow visualization of the target tissue.
Ab interno viscocanulostomy/canaloplasty is a procedure that dilates the Schlemm canal, using a viscoelastic material injected through a goniotomy opening, and can be accomplished with an illuminated microcatheter (iTrack; Ellex iScience, Inc., Fremont, CA, USA) or a modified ab interno filament trabeculotome (Visco 360, Omni720; Sight Sciences, Inc. Menlo Park, CA, USA). There is no evidence in the literature on the effect of ab interno canaloplasty in the pediatric glaucomatous eye, and in most childhood glaucoma cases, ab interno canaloplasty likely offers no theoretical advantages over goniotomy when performed alone. However, as both the illuminated microcatheter and the ab interno filament trabeculotome can be used to perform ab interno trabeculotomies, the effect of adding viscocanulostomy/canaloplasty to goniotomy/trabeculotomy remains uncertain. The iStent trabecular bypass stent (iStent; Glaukos, San Clemente, CA, USA) offers no theoretical advantage over goniotomy.
Angle Open and Previously Incised, Cornea Clear
There has been a recent resurgence of interest in the surgical anastomosis of the anterior chamber and the suprachoroidal space as a means of lowering IOP. Ab interno suprachoroidal shunts such as the CyPass Micro-Stent (Alcon, Inc. Fort Worth, TX, USA) and iStent Supra (Glaukos; not FDA approved) may offer blebless alternatives to filters or GDDs in pediatric patients after circumferential angle surgery has failed. As of August 2018, there is a global voluntary recall of the CyPass device because the group that received the implant had increased endothelial cell loss compared to the control group, though the clinical significance of this finding remains uncertain.14 None of the subconjunctival or suprachoroidal devices on the market are likely to out-perform the standard filter or tube shunt. The Xen gel stent (Allergan, Dublin, Ireland) is an ab internally placed channeling device that allows the shunting of aqueous from the anterior chamber to a perilimbal bleb. In eyes with open angles that have failed angle surgery, Xen gel stent may offer an alternative to traditional trabeculectomy and/or tube shunt, with the theoretical advantage of a fixed channel size and thus a decreased risk of hypotony, although the long-term risk/benefit profile over traditional trabeculectomy augmented with mitomycin C in children is unknown.
Angle Closed
When children present with angle closure and elevated IOP, as in some cases of glaucoma following cataract surgery or glaucoma associated with retinal ablation for retinopathy of prematurity, surgical options are traditionally limited to either a filtering procedure or the implantation of a GDD. With recent advances in microsurgical instrumentation, however, goniosynechialysis (GSL) under direct gonioscopic visualization can offer IOP control in angle-closure glaucoma without resorting to GDD implantation or bleb creation. GSL can be performed as a standalone procedure in angle-closure IOP elevation or combined with lensectomy and/or endoscopic cyclophotocoagulation (ECP). The latter is appropriate if a large or abnormally shaped lens is determined to play a role in the etiology of angle closure. During GSL, the pupil is first constricted by administering an intracameral miotic solution. Through a clear corneal or corneoscleral incision, intraocular microforceps is advanced across the anterior chamber to the opposite angle. Under direct gonioscopic visualization, the microforceps is used to gently peel the anterior synechiae open, usually until the ciliary body band can be visualized. Once the trabecular meshwork is visible, additional angle treatment such as goniotomy or ab interno trabeculotomy can be performed to enhance the outflow facility. ECP is the only MIGS that does not require direct visualization of the angle, owing to the endoscopic camera system (Endo Optiks Laser System; Beaver-Visitec International, Waltham MA, USA). To perform ECP, the endoscope (equipped with an co-axial camera, light source, aiming beam and diode laser, wavelength 810 nm) is advanced through a wound on the clear cornea, anterior sclera, or the pars plana until the ciliary processes are visible on the view screen. Low-energy laser is applied to the ciliary processes until whitening and shrinkage occur (without any overt cavitation bubble formation). In one modest-size series of children with glaucoma following cataract surgery, ECP resulted in a success rate of 54% after a mean of 7.2 years' follow-up.15
Summary
MIGS may offer robust alternatives to enhance the traditional surgical paradigm in the treatment of childhood glaucoma. Ab interno angle procedures may be offered if the angle is open and unincised, and the cornea allows the direct gonioscopic visualization, whereas goniosynechialysis may be a useful procedure, with or without ECP, for eyes with angle closure. As with all novel procedures without established, longstanding safety profiles, MIGS procedures must be applied judiciously in children, as even minor complications can compound into major ones over the length of the young patients’ lives.
References
- Papadopoulos M, Edmunds B, Chiang M, et al. Section 5: glaucoma surgery in children. In: Weinreb RN, Grajewski A, Papadopoulos M, Grigg J Freedman S, eds. World Glaucoma Association Consensus Series – 9: Childhood Glaucoma. Amsterdam, The Netherlands: Kugler Publications; 2013:95-136.
- Jayaram H, Scawn R, Pooley F, et al. Long-term outcomes of trabeculectomy augmented with mitomycin C undertaken within the first 2 years of life. Ophthalmology. 2015;122(11):2216-2122.
- Sood S, Beck AD. Cyclophotocoagulation versus sequential tube shunt as a secondary intervention following primary tube shunt failure in pediatric glaucoma. J AAPOS. 2009;13(4):379-383.
- Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC; Tube Versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804-814.e1.
- Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96-104.
- Hodapp, EA. Personal Communication. June 15, 2018. Bascom Palmer Eye Institute, Miami, FL USA.
- Barkan O. Technic of goniotomy for congenital glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1948;52:210-226.
- Bothun ED, Guo Y, Christiansen SP, et al. Outcome of angle surgery in children with aphakic glaucoma. J AAPOS. 2010;14(3):235-239.
- Bowman RJ, Dickerson M, Mwende J, Khaw PT. Outcomes of goniotomy for primary congenital glaucoma in East Africa. Ophthalmology. 2011;118(2):236-240.
- Mandal AK, Chakrabarti D. Update on congenital glaucoma. Indian J Ophthalmol. 2011;59(1): S148–S157.
- Grover DS, Smith O, Fellman RL, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol. 2015;99(8):1092-1096.
- Grover DS, Fellman RL. Gonioscopy-assisted Transluminal Trabeculotomy (GATT): Thermal Suture Modification With a Dye-stained Rounded Tip. J Glaucoma. 2016;25(6):501-504.
- Mendicino ME, Lynch MG, Drack A, Beck AD, Harbin T, Pollard Z, et al. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. J AAPOS. 2000;4(4):205-210.
- Alcon, Inc. Alcon announces voluntary global market withdrawal of CyPass Micro-Stent for surgical glaucoma. https://www.novartis.com/news/media-releases/alcon-announces-voluntary-global-market-withdrawal-cypass-micro-stent-surgical-glaucoma. Accessed April 17, 2019.
- Cantor AJ, Wang J, Li S, Neely DE, Plager DA. Long-term efficacy of endoscopic cyclophotocoagulation in the management of glaucoma following cataract surgery in children. J AAPOS. 2018;22(3):188-191.