Introduction
The etiology and genetics, pathophysiology, diagnosis, and management of Stargardt disease, Best disease, oculocutaneous albinism, and X-linked retinoschisis are discussed in this chapter.
Stargardt Disease
Stargardt disease (STGD1, other names used: juvenile macular dystrophy, fundus flavimaculatus) is the most common inherited macular dystrophy. It is caused by mutations in the ABCA4 gene and has an estimated prevalence of 1/8,000–1/10,000.1-2 The condition owes its name to the German ophthalmologist Karl Stargardt, who first described it in 1909.2 Known for its high phenotypic and genotypic heterogeneity, Stargardt typically (with the exception of foveal-sparing forms) causes progressive deterioration of retinal structure, as well as vision loss, in children and young adults, incurring a substantial socioeconomic and psychological burden.
Etiology/Genetics
STGD1, caused by mutation in ABCA4, is inherited in autosomal recessive manner. There are known autosomal dominant forms of Stargardt-like dystrophies associated with mutations in the EVOL4 and PROM1 genes.3 ABCA4 is a large photoreceptor-specific gene with 50 exons. It had been subjected to intense genetic analysis and more than 900 pathogenic variants had been detected to date.4,5 Because of this, there is a vast clinical variation among patients with STGD1 (age of onset, rate of progression, severity). Genotype-phenotype correlation remains very challenging.
Pathophysiology
Briefly, ABCA4 encodes an ATP-binding cassette transporter, which is localized to the rims of the photoreceptor outer segment disks, both in rods and in cones. This rim protein is involved in transport of vitamin A derivatives. Specifically, the rim protein transports N-retinylidene-phosphatidylethanolamine (N-RPE) that is produced from 11-cis-retinal at the initiation of phototransduction. N-RPE is transported from the disc space into cytoplasm, where it is reduced and then transported to RPE for recycling back to 11-cis-retinal. In the presence of dysfunctional rim protein, the N-RPE accumulates and leads to formation of compounds toxic to RPE cells, such as lipofuscin and N-retinylidene-N-retinyl-ethanolamine (A2E).5 The RPE and photoreceptors are metabolically interdependent. Disturbed or destroyed RPE cells lead to the death of photoreceptors. 5
History
A careful review of development of vision symptoms is important. The early symptoms of juvenile macular dystrophy are variable and not specific. Among the 54 patients with confirmed biallelic ABCA4 Stargardt disease followed by us at Boston Children’s Hospital, the onset of symptoms falls within an age range of 7 years to 10 years (54 subjects). ProgSTAR, an international retrospective and prospective multicenter study conducted from 2013–2019, in their report of early onset Stargardt disease (51 subjects), describes the mean age of first symptoms as 7.2 years.1 At this age, patients are able to communicate difficulties with vision. Many patients are identified based on failed school vision screening. In addition to the onset and duration of the symptoms, the prior care of the individual as well as the family history of visual problems must be obtained. Retrieving past records, if any, can also be helpful.
Signs and Symptoms
Typical first visual complaints include: failed screening test at school or in the pediatrician’s office, difficulties seeing from the board, difficulties reading, abnormal visual behavior as noticed by teachers and parents (coming too close to a television or computer, holding things too close to one’s face, or not seeing things that are coming toward them, such as a hockey puck or a ball), no observed improvement with glasses prescription, and abnormal eye movements. Older children (10-12 years) may describe peculiar visual phenomena such as blurriness in certain areas of visual fields, central scotomas, or spots on the white background. Importantly, in some cases a missed diagnosis can lead to rising frustration in children who may be falling behind their peers in school or kindergarten, or who experience a drop in reading level. This can lead to manifestation of unsettling psychological, behavioral, and motor problems and, at times, to incorrect diagnoses of attention-deficit/hyperactivity disorder (ADHD) or dyslexia.
Younger siblings of STGD1-diagnosed patients are often brought for evaluation very early, sometimes in infancy. In our opinion, they should be offered an opportunity for genetic diagnosis and they should also be monitored for development of symptoms. Not only does this position providers to act in a timely manner, it also contributes to further understanding of the disease.
Physical examination
A comprehensive pediatric ophthalmic examination should be performed. That includes age-appropriate visual acuity testing, recording the power of existing glasses (if any), stereopsis and binocular vision testing (if possible), documenting motility and ocular alignment, external and anterior segment evaluation, intraocular pressure examination, visual field testing (if age-appropriate), performing a dilated fundus examination with indirect ophthalmoscopy, optical coherence tomography (OCT; if possible), and cycloplegic refraction.
By ophthalmoscopy, fundus appearance may be normal at early stages. Because of Stargardt’s heterogeneity, retinal imaging and ancillary diagnostic studies are important. (see Imaging, ancillary studies and diagnosis) Best corrected visual acuity (BCVA) ranges from 20/25 at early stages to as low as 20/800 in advanced stages. The ProgStar study estimates that the median time to develop severe visual impairment (acuity poorer than 20/200) is 12 years.1,6 More than half of children with STGD1 fall into the moderate to severe visual impairment category, if classified by visual acuity alone.
Typical fundus examination findings are central macular atrophy and yellow-white deposits--“flecks”--in the central and midperipheral retina (Fig 1A). Disturbance of ocular motility may or may not be present at early stages, but it is observed quite often later in the course. With progressive macular atrophy and development of central scotoma, unstable and eccentric fixation ensues.7
Imaging, ancillary studies and diagnosis
Recommended imaging modalities for diagnosis are fundus photographs, fundus autofluorescence (FAF) images, SD-OCT, and electroretinography (ffERG). Color fundus images will demonstrate the atrophic lesions, pigment alteration, and typical flecks. On corresponding FAF images, macular lesions have a decreased autofluorescence signal demarcating the atrophic macular area. Active flecks visible on fundus examination (lipofuscin accumulation) appear as hyperfluorescent spots; resorbing, old flecks (RPE atrophy and photoreceptors loss) appear hypofluorescent.8 On macular SD-OCT, there is a progressive ellipsoid zone disruption and outer nuclear layer loss (Fig 1A, B, C). However, all the above is usually not present on early stages of the disease, and the severity and extent of lesion varies between subjects. Fishman and colleagues (1976) recognized the progressive changes in phenotype.9 Cukras and colleagues (2012) noted progressive radial foveal-to-peripheral extension of flecks at rates of 14–58 μm per month.8 Possibly, this spatial distribution is a manifestation of the underlying molecular mechanisms.
Electroretinographic (ERG) responses can show a range of abnormalities. Some authors classify the patients with Stargardt based on ERG results, grouping them into normal, minimal, or severe dysfunction;10 or normal scotopic/reduced photopic; or both abnormal scotopic and photopic responses groups.11 To date, however, no correlation between ERG changes, clinical features, or genotype has been established. Sample ERG responses are illustrated in Fig. 1D. None of the proposed classifications, whether based on fundoscopic findings or on ERG responses, have been widely adopted in clinic.
Stargardt disease should be differentiated from the other cone-rod dystrophies. A final diagnosis is only secured by genetic testing. In the absence of fundoscopic changes, early diagnosis of Stargardt is quite challenging. Among our patients with STGD1, the average time between first signs and established diagnosis approaches 3 years. Having an established diagnosis is important for families and clinicians for proper monitoring and guidance.
General management and prognosis
Typically, visual acuity and central vision decline progressively over the course of the disease, although some studies report that vison stabilizes at the 20/200 level.2 In advanced stages, an absolute central scotoma (Fig. 1E) and eccentric fixation develop.
In the absence of effective treatment, management is focused on monitoring the disease and providing the optimal accommodations and arrangements for school and/or work (eg, magnifying devices and applications, enhanced vision TV devices, and computers), social and psychological support, and occupational counseling. A low-vision specialist consultation is recommended. Teaching Braille as well as using an orientation and mobility service should also be considered. Genetic counseling is recommended.
Therapeutic strategies
There is no definitive treatment available at the moment. Several strategies are being investigated; some have reached clinical trials. This includes pharmacologic interventions (NCT03772665, NCT04489511, NCT03364153, NCT02402660), stem cell therapy (NCT03011541). The pharmacologic strategies are primarily focused on reducing toxic retinoids, as follows:
-
Emuxistat hydrochloride inhibits RPE65 and reduces 11-cis-retinal production (NCT03772665). A preliminary report indicates biological activity in STGD1 patients.12
-
STG-001 (Stargazer Pharmaceuticals) reduces the retinol-binding protein (RBP4) concentration in plasma and therefore reduces vitamin A uptake and retinoid production (NCT04489511).
-
ALK-001 (Alkeus Pharmaceuticals), chemically modified deuterated vitamin A designed to substitute vitamin A and reduce retinoids formation (NCT02402660)
Complement-mediated therapeutics for STGD1 are being explored, as they are for AMD and geographic atrophy (NCT03364153 - complement C5 inhibitor). The gene replacement therapy trial was terminated by the sponsor, not for safety reasons, but for lack of efficacy. The enrolled participants who received gene replacement are being followed (NCT01367444, NCT01736592).
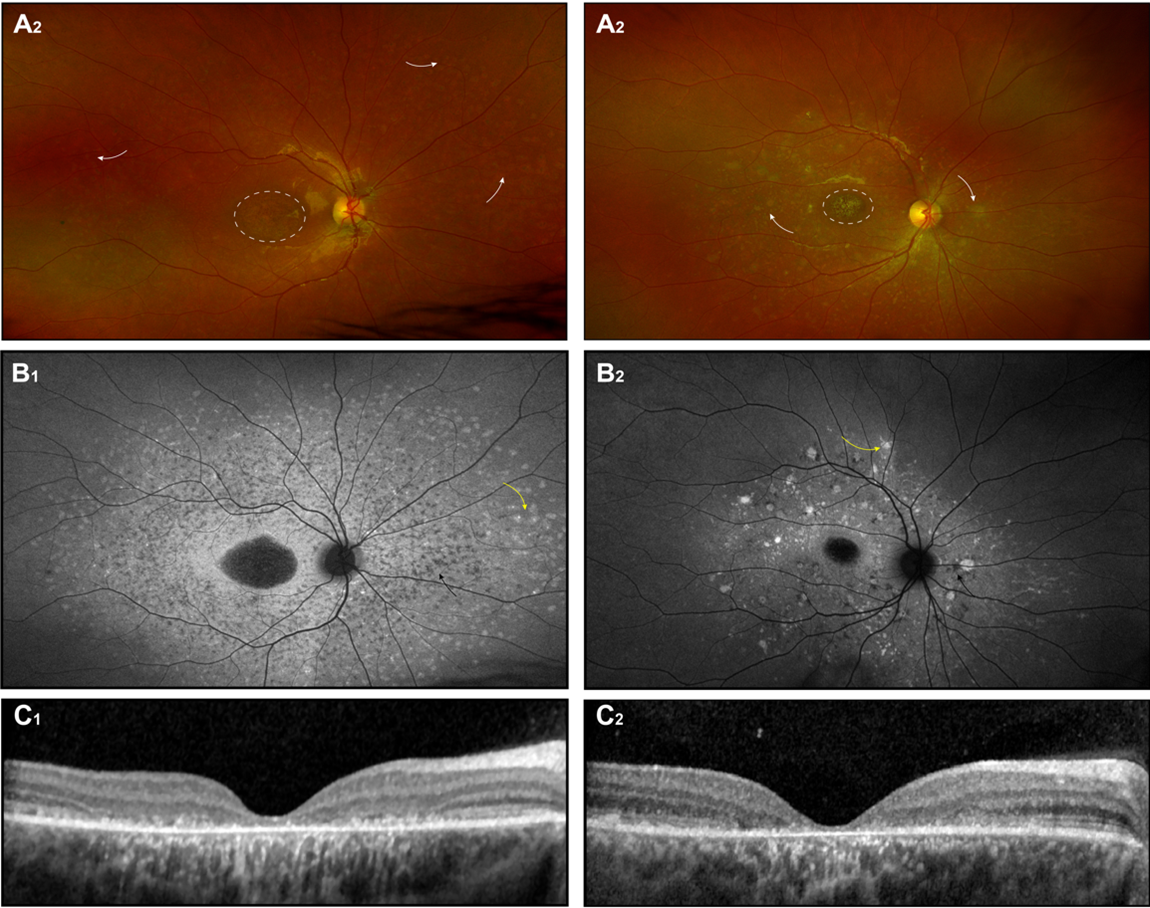
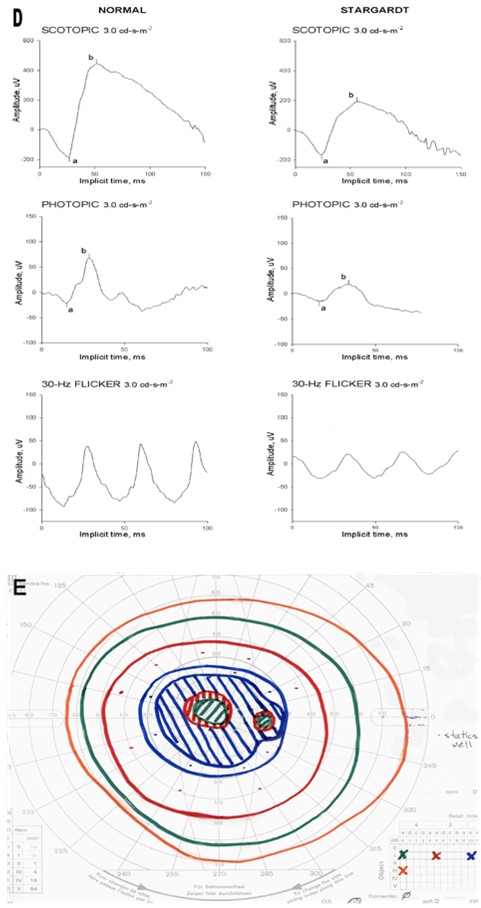
Figure 1. Stargardt disease. Fundus photograph(A), autofluorescence photograph (B), OCT images (C) of 2 unrelated patients with biallelic ABCA4 mutations; Each patient became symptomatic at ~ 6.5 years, the images shown were obtained at age of ~ 12 years (D)(E) The ERG records and results of Goldman perimetry for patient 1 at age 12 years are also shown.
Best Disease (Juvenile Vitelliform Macular Dystrophy)
Disease
Best vitelliform macular dystrophy (BVMD), also known as Best disease, is a rare, progressive, early onset retinal dystrophy. BVMD is the most common of the 5 distinct retinal degenerative diseases associated with mutation in the BEST1 (VMD2) gene (“bestrophinopathies”).13 The name originates from another German ophthalmologist, Fredrich Best, who first described a family with this macular dystrophy in 1905.14 The exact prevalence remains unknown. Most studies describe White or northeastern European populations. Previous estimates of Best dystrophy incidence show 2 in 10,000 in Sweden, and 1.5 in 100,000 in Denmark. The recent study of the Olmsted County population (Minnesota) was the first to report BVMD prevalence in the US and showed that it affects 1 in 16,500 to 1 in 21,000 individuals in this area.14-17
Etiology
BVMD is inherited mainly in an autosomal dominant mode; incomplete penetrance and a variable broad phenotype may be observed.18 The responsible gene resides on chromosome 11q12-13 and contains 11 exons; more than 200 mutations VMD2 are identified to date (up to 300 according to some sources).19-20 Within the eye, VMD2 is exclusively expressed in RPE, which is critical for retinal development and maintenance. Mullins and colleagues demonstrated (by immunohistochemistry, western blot, and quantitative PCR) the higher bestrophin expression in peripheral than in the macular regions.21 This nonuniform expression leads to the hypothesis that if there is a loss of function in BEST disease, peripheral RPE compensates and functions normally with 1 working VMD2 copy, while in the macula RPE becomes insufficient, which leads to development of the typical macular lesion.21 Alternatively, the specialized foveal/parafoveal structures may account for the predominance of macular lesions.
Pathophysiology
BEST1 encodes bestrophin, an integral transmembrane protein with multifaceted mode of action. It forms a calcium-dependent chloride and bicarbonate channel regulating the ion transport. In addition, it acts as an inhibitor of voltage-dependent intracellular calcium channels. There is evidence that the BEST1 gene has a role in normal ocular development. While the exact mechanism of its influence on eye development remains unclear, there are reported abnormalities—such as microcornea, rod-cone dystrophy, early onset cataract, and posterior staphyloma—that may arise in association with specific VMD2 mutations.19,22,23 The current understanding of the pathophysiologic mechanism may be summarized in the following way: With the altered ion and fluid transport that a bestrophin mutation causes, the RPE becomes dysfunctional and disrupts RPE microvilli and their interactions with photoreceptor outer segment, producing a serous neuroretinal detachment. Impaired phagocytosis leads to subretinal accumulation of lipofuscin and cellular debris responsible for the classic vitelliform, “egg-yolk” lesions.19-20
History
The family history of a majority of children with Best disease is significant. Thus, a clinical history should include any family members with “eye problems” or a known diagnosis of vitelliform dystrophy. In fact, affected parents, aware of the heritable nature of the disorder, may bring their child for the eye examination before any symptoms are observed. On the other hand, a negative family history does not exclude the diagnosis, given the variability of clinical presentation and age of onset, as well the fact that 7% to 9% of people with BEST1 mutations have normal acuity.13 The disease can be caused by de novo mutation as well.14,24,25 The condition may go undetected until later in life, especially if visual acuity is little affected. The usual age of onset is before puberty, typically between 3 and 15 years of age with a mean of 6 years, according to some authors.19,24
Signs and Symptoms
The major complaint, if any, at the time of first ophthalmologic evaluation is decreased or blurred vision. Metamorphopsias and central vision deficits may be reported. While the main phenotypic findings are at the posterior pole and quite distinct, acuity may be normal at the time of diagnosis. Many patients show different stages of the disease in each eye and vision loss is often asymmetric (clinical stages described below).19,26 Visual acuity ranges between 20/20 and 20/200, depending on clinical stage, and it may be poorer if complications (eg, choroidal neovascularization, hemorrhage) develop. Notably, significant correlation of patient age with acuity, as well as disease stage with acuity and central scotoma size, has been described.26,27
Physical examination
A complete pediatric ophthalmic examination should be performed including age-appropriate visual acuity test, recording the power of existing glasses (if applicable), stereopsis and binocular vision testing (if possible), motility and ocular alignment, external and anterior segment evaluation, intraocular pressure, visual field test (if age-appropriate), dilated fundus exam and cycloplegic refraction. The presence of strabismus and high refractive error may impact management. Esotropia, hyperopia, and amblyopia may be noticed. There are reported anterior chamber abnormalities associated with BEST disease, such as shallow anterior chamber, closed/narrow anterior chamber angle, and angle-closure glaucoma.28 Wittstrom and colleagues described 3 adults and 1 child with BVMD; the findings were small to moderate hyperopia (2–3 diopters) in adults and high hyperopia (8–9 diopters) in a 10-year-old child, as well as shorter axial lengths in all:28 So-called axial hyperopia is a known risk factor for development of primary angle closure.29
Best’s hallmark finding on the fundi exam is “egg-yolk” macula lesion--solitary or multifocal,30 circular, well-circumscribed, yellow-opaque, 0.5-2-disk-diameter patches. (Fig 2A)
Best vitelliform dystrophy is classified into 5 clinical stages:13, 28,31,32
-
Previtelliform - normal vision and fundus appearance; subtle RPE alterations.
-
Vitelliform - vitelliform “egg-yolk” lesions; vision is normal or mildly decreased
-
Pseudohypopyon - layering of deposited material; no major changes in vision
-
Vitelliruptive - material disruption/partial resorption, “scrambled-egg” appearance; worsening of vision
-
Atrophic - chorioretinal atrophy, scarring; vision is markedly reduced (20/200)
Stages III and IV stages may be reversed in order in the literature: pseudohypopyon follows vitelliruptive stage, as when the vitelliform material breaks, the horizontal fluid level develops.14,19 Neovascularization and leakage are interchangeably discussed as both stage V or a separate stage VI, with a reported occurrence of 2%–9% of Best dystrophy cases.19
Imaging, ancillary studies, and diagnosis
Imaging could be critical for early and accurate diagnosis. Fundus photographs, fundus autofluorescence images, and OCT are recommended.
Fundus photography demonstrates a classical macular lesion(s), or a normal fundus appearance at early stage. On OCT, there is neuroretinal detachment with hyperreflective material and hyporeflective fluid between photoreceptors and RPE: Atrophic scarred areas appear as prominent hyperreflective structures.13,15,19 (Fig 2 A, C)
Fundus autofluorescence images display intensely increased autofluorescence signal of the vitelliform lesion. (Fig 2B) The hyperfluorescent signal pattern evolves as the disease progresses, showing dispersed granulation at the vitelliruptive stage and decreased to absent signal at the atrophic stage. The fluorescein angiogram (FA) reveals an opposite pattern, from hypofluorescence in the area covered by the lesion at early stages, changing to partially blocked fluorescence of the vitelliruptive stage, to hyperfluorescence at atrophy and neovascularization/leakage stages.14,19
Since administration of intravenous dye is not readily accomplished in some pediatric patients, FA may be deferred or completed during examination under anesthesia. OCT angiography (OCT-A), should be considered. It is a noninvasive technique that allows good visualization of retinal and choroidal vasculature. Reported OCT-A abnormalities in BVMD include foveal avascular zone distortion, patchy vascularity loss in deep and superficial layers of retina, and net and ring-shaped choroidal neovascularization patterns if the latter develops.33,34
Historically, an abnormal electro-oculogram (EOG) was the criterion for the diagnosis. The characteristic for Best dystrophy is an abnormally low ratio of potentials recorded between cornea and retina in the light- and dark-adapted eye (Arden ratio). EOG is abnormal in all stages,13,18,19 while ERG is normal or nearly so. With the availability of genetic testing, electrophysiologic testing is not critical for diagnosis.
While BVMD fundus appearance along with family history may be quite distinct, the differential diagnosis should include central serous chorioretinopathy, infectious retinochoroiditis, Stargardt disease with large flecks, large dehemoglobinized subretinal hemorrhage, and other retinal degenerations. Again, specific diagnosis is usually secured by genetic testing.
Therapeutic strategies
There is no curative treatment and no clinical practice guidelines are available. Gene, pharmacologic, and stem-cell based therapies are under investigation. Complications, should they develop, are treatable. For instance, choroidal neovascularization is treated with intravitreal anti-VEGF injections, laser photocoagulation, and photodynamic therapy.29,35-38 A rarely reported complication--macular hole with or without detachment--is managed surgically.39-41
General management and prognosis
The visual prognosis can be considered guarded, although there is typically a very slow decline in visual acuity over decades, reaching the visual impairment level, if at all, relatively late (in the fourth and fifth decades). According to some references, best corrected visual acuities at different stages are 20/20 in pre-vitelliform stage, 20/20 to 20/60 in vitelliform and pseudohypopyon stages, 20/20 to 20/120 in vitelliruptive stage, and 20/200 or worse at atrophic and choroidal neovascularization stages.14,42
In addition to general management and support provided for nearly all patients with inherited retinal disease (see Stargardt Disease section), children with BVMD are monitored for complications and amblyopia. Genetic counselling to discuss the nature of the disease, inheritance mode, and potential risks to offspring is recommended to young adults.
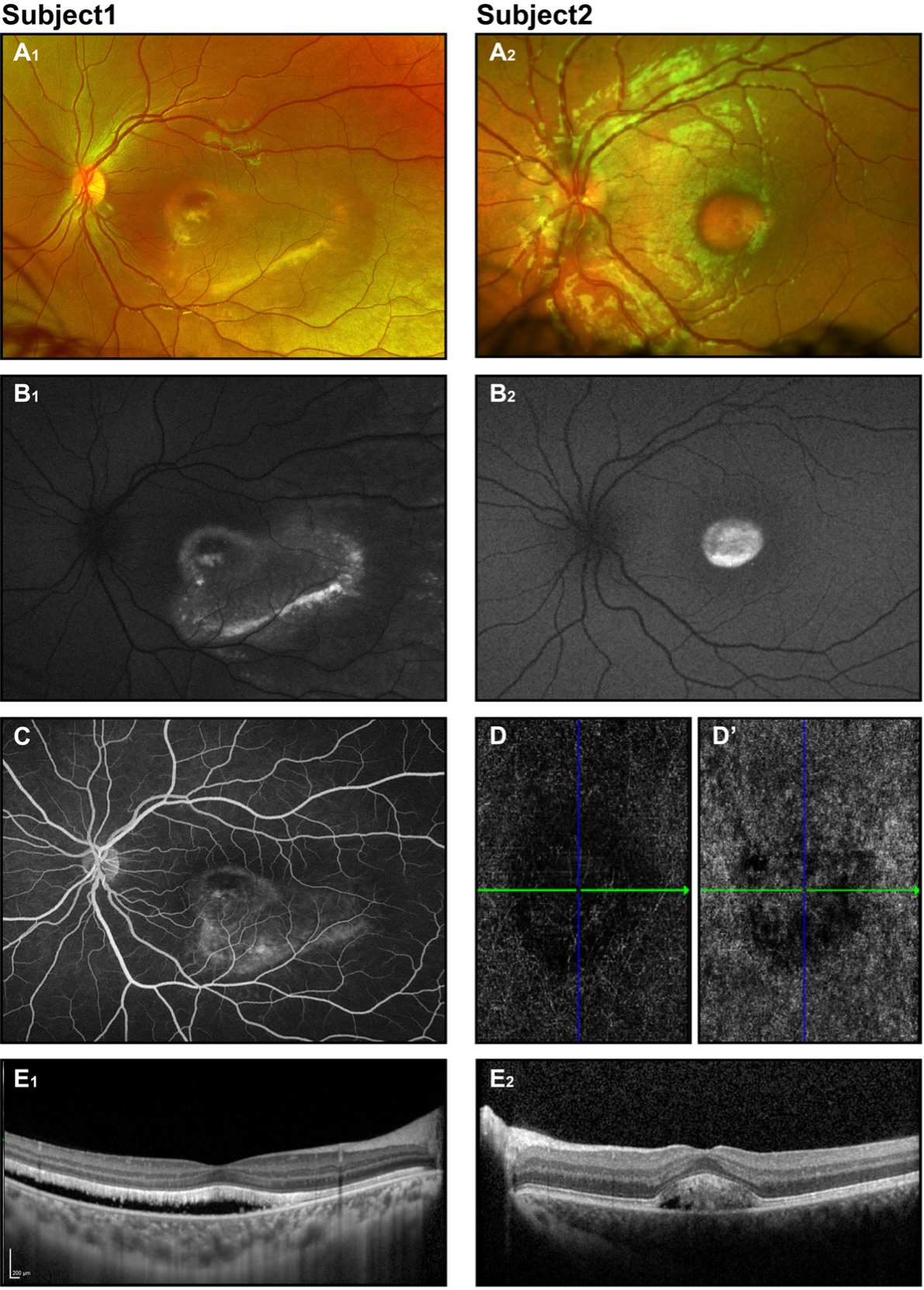
Figure 2. Best Disease. Retinal imaging of 2 subjects with BEST disease (8.7 and 6.3 years old). Color fundus photographs show vitelliform macular lesions (A1, A2), with increased signal on corresponding FAF images (B1, B2) - likely vitelliruptive and pseudohypopyon stages respectively for subj 1 and 2. Fluorescent angiogram completed for subj 1 (C) showed no leakage. OCT angiogram obtained for subj 2 (D, D’)- presented avascular and choriocapillaris layers show no CNV. On OCT (E1, E2) neuroretina detachment is illustrated.
Oculocutaneous albinism/foveal hypoplasia
Disease
Albinism (from Latin albus = white) is a group of heterogenous and phenotypically variable genetic disorders of melatonin biosynthesis. The estimated overall prevalence in the Western world is 1:17,000 to 1:20,000.43 It is divided into oculocutaneous albinism (OCA) and ocular albinism (OA). OCA has distinctive gross and microscopic features of skin, hair, and eyes. OA has grossly visible features of the eyes and only microscopic features (macromelanosomes) of the skin. Common ocular features of both OCA and OA include reduced vision, hypopigmentation of the retina, iris translucency, photophobia, foveal hypoplasia, nystagmus, relative optic nerve hypoplasia, abnormal neuronal wiring (abnormal decussation of nerve fibers at the optic chiasm), and strabismus.43-45
Etiology/Genetics
There are at least 7 genes associated with isolated OCA; all inherited in an autosomal recessive fashion. Four out of 7 (OCA1-4) are well characterized (by genes TYR, OCA2, TYRP1, SLC45A2). OCA1A, which is caused by 2 null TYR mutations and complete inactivity of tyrosinase, is the most severe form of OCA; there is a complete lack of melanin production throughout life. Milder forms have some melanin accumulation over time.46-49 With over 500 reported OCA mutations, some OCA cases remain genetically unexplained.47
In contrast to OCA variety, there is a single gene associated with ocular albinism, GPR143, and that disorder is inherited in an X-linked recessive manner.45,49
Pathophysiology
In a nutshell, the albinism-causing mutations result in deficient melanin production. For instance, TYR-encoded tyrosinase regulates the melanin biosynthesis pathway, from L tyrosine to L-DOPA to dopaquinone. The OCA2 gene encodes for integral melanosomal protein (P-protein) necessary for building melanosomes, the specialized melanin-producing organelle involved in pigment production. The TYRP1 gene product, tyrosinase-related protein-1, stabilizes tyrosinase. Mutations in TYRP1 cause a delayed tyrosinase maturation and early degradation. SLC45A2-encoded protein is thought to be a membrane transporter in melanosomes.47,50 In ocular albinism (OA) the G-protein coupled receptor 143 gene (GPR143) encodes for proteins important for the development and maturation of melanosomes.51
Foveal hypoplasia is a common phenotypic feature of all forms of albinism, although it is not specific to albinism. The mechanisms underlying a lack of foveal development have yet to be elucidated. While there is a recognized role of melanin in the control of development, not only ocular structures but also of the visual pathways (abnormal projection of part of the temporal retina to the visual cortex contralateral to the eye in albinism), the mechanisms accounting for these anomalies remain incompletely specified.44,52,53 Investigations have been conducted on the effect of melanin on spatial-temporal properties of retinal ganglion cell (RGC) distribution and on formation of the optic pathways. Disrupted melanin biogenesis is thought to affect the pace of RGC production, differentiation, and proper proportion between those projecting to the same and those projecting to the opposite side of the brain. The normal routing of retinal fibers is thought essential for the binocular vision.52-56 A number of studies focused on foveal cone specialization, but there appears to be no consensus regarding the albino fovea; various degrees of foveal maturation and both decreased and increased central cone densities were reported.57-59
History
With phenotypic variation among the types of albinism, the most dramatic cutaneous traits occur in OCA1A and are recognized easily by the family. An individual with albinism has white hair, white lashes, white skin, and pink eyes. In other forms of albinism that have some pigmentation and in ethnic groups with relative hypopigmentation, a comparison with other family members is pivotal.60 Yet, the most bothersome feature to the parents of children with albinism is nystagmus. As a result, many patients may initially present to neurology or neuro-ophthalmology. Detailed description and history of nystagmus should be learned, including age of onset, amplitude, frequency, direction, changes with time, and video recordings of eye movements (when/if possible). Birth, prenatal course, and exposures should also be reviewed. An MRI of the brain and orbits is frequently ordered to look for evidence of associated neurological abnormalities.61
A careful patient history should be collected to ascertain features of hypopigmented syndromes. Please see the differential diagnoses section.
Signs and Symptoms
The major complaints prompting an office visit are nystagmus, decreased vision, and photophobia. Nystagmus develops during first few weeks of life and typically lessens with age. Acuity development goes forward approximately parallel to, but lower than, normal.44 The degree of pigmentation may affect the acuity: the lower the pigmentation, the lower the acuity. As nystagmus decreases, visual acuity improves.44 Reported VA estimates for the most common OCA forms are 20/100 to 20/400 for the most severe OCA1A, 20/100 to 20/200 for OCA1B (aka “yellow OCA”), 20/60 to 20/100 for OCA2, and 20/100 to 20/200 for OCA4.60
Physical examination
A comprehensive ophthalmic examination should be performed. Pendular nystagmus with postural head changes is often seen. An increased incidence of strabismus is described in children with OCA, and may be related to abnormal decussation of optic nerve fibers.44 In addition to reduced adnexal and cutaneous pigmentation, which varies widely, decreased iris pigmentation is noted. Iris sites that transilluminate light are observed, from subtle to quite profound changes with the edge of the eye lens visible. A detailed slit-lamp examination should be completed if possible. Anterior segment dysgenesis (posterior embryotoxon, Axenfeld anomaly, aniridia) is reported for foveal hypoplasia-associated syndromes.62,63 Ophthalmoscopy reveals a lack of foveal reflex and translucent, yellow-looking retina with prominent choroidal vessels (Fig. 3). The optic disc should be examined for concomitant optic nerve hypoplasia.
Imaging, ancillary studies and diagnosis
With clear, unambiguous results of physical examination findings, no additional imaging is required for diagnosis of a form of albinism. However, color fundus photographs and especially OCT may be obtained—not only for documentation purposes but also for evidence in ambiguous cases. These are especially helpful for OCA types with some preserved pigmentation when clinical presentation is not convincing for OCA. OCT, being a challenging test to complete for a young patient with nystagmus, may demonstrate foveal hypoplasia and preserved inner retinal layers (variable loss of normal foveal architecture is reported).45,47 Full-field ERG amplitudes, if recorded, generally fall within the range of normal. Supernormal amplitudes have been reported.64-65
The diagnosis is often established based on clinical skin and ocular findings. The specific diagnosis is confirmed with genetic testing. OCA should be distinguished from syndromes associated with melanin production defects. These include: 43,50,60
-
Hermansky-Pudlak syndrome (HPS): A rare disorder, although global occurrence is notable; affected individuals mainly in Swiss and Puerto Rican populations have been identified. The features of HPS are bleeding diathesis, neutropenia, lung fibrosis, granulomatous colitis.
-
Angelman and Prader-Willi syndromes: A chromosome 15q11-13 microdeletion, paternal or maternal; neurodevelopmental disorders that could be associated with hypopigmentation.
-
Waardenburg syndrome: An autosomal dominant disorder of the MITF gene with sensoneural hearing loss, skin hypopigmented areas, iris stromal atrophy/heterochromia.
-
Chedyak-Higashi syndrome: A rare disorder characterized by susceptibility to bacterial infections, coagulation defects, progressive neurologic abnormalities and hypopigmentation.
-
Griscelly syndrome: A rare disorder of skin pigmentation, silver grey hairs; could be associated with neurologic abnormalities and roving eye movements.
-
Tietz-albinism-deafness syndrome: An autosomal dominant MITF-related disorder with profound sensorineural hearing loss and hypopigmented fundi but no vision impairment.
Differential diagnosis should include the disorders associated with early onset nystagmus. Among those are FRMD7-related infantile nystagmus, Cockayne syndrome, spasmus nutans, optic nerve atrophy and hypoplasia, achromatopsia, and aniridia.
Therapeutic strategies
There is no cure for albinism. In pre-clinical work, nitisinone (a drug that inhibits the metabolism of tyrosine and is FDA approved for the treatment of tyrosinemia) was shown to improve fur pigmentation and ocular pigmentation in the mouse model. A one-year pilot study in humans, however, did not document increase in skin, iris, or hair melanin.66 Another investigative compound, L-Dopa, did not result in improved visual acuity in a randomized trial of 45 patients.67 The potential of gene therapy is being explored.68
General management and prognosis
Albinism is a static condition with no progressive vision decline and no effect on intelligence. Prognosis usually refers to increased risk of skin cancer and socioeconomic issues. Management is focused on lifelong sun protection, regular ophthalmic evaluations, and monitoring (correcting the refractive errors, managing of amblyopia and strabismus if developed, occasional nystagmus surgery). Low vision evaluation and recommendations are helpful as well. Genetic testing and counseling are encouraged for the family.
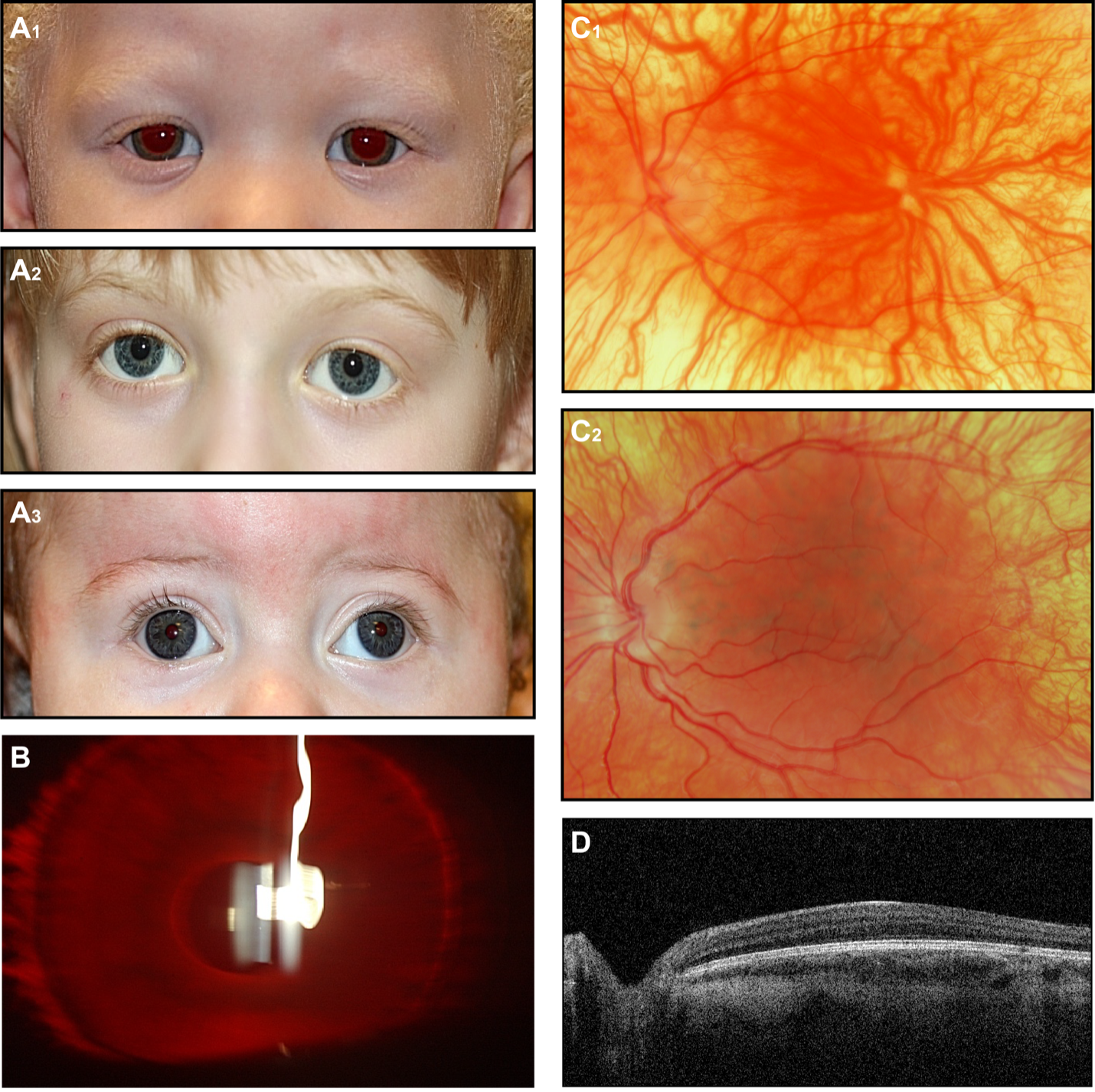
Figure 3. OCA (A) External photographs of subjects with clinical diagnosis OCA(A1), genetically confirmed OCA2 (A2) and OCA4 (A3). (B) Slit-lamp photograph of the OCA subject demonstrates iris transillumination. (C1)(C2) fundus photographs of 2 individuals with clinical OCA diagnosis. (D) OCT demonstrates the absence of foveal depression and preserved laminae (the image is taken from subject presented on C2 fundus photo).
X-Linked Retinoschisis
Disease
X-linked juvenile retinoschisis (XLRS) is the leading cause of hereditary juvenile macular degeneration in boys, accounting for approximately 5% of all childhood-onset, inherited, progressive retinal dystrophies. The prevalence of XLRS is estimated to be 1 in 5,000 to 25,000 men worldwide.69
Etiology/Genetics
XLRS is a monogenic disease caused by mutation of the RS1 gene, which is located on the short arm of the X-chromosome and encodes the extracellular binding protein, retinoschisin. XLRS is inherited in an X-linked recessive pattern. In males, 1 altered copy of the gene in each cell is sufficient to cause the condition. In females, a mutation would have to occur in both copies of the gene to cause the disorder. Because it is unlikely that females will have 2 altered copies of this gene, males are affected by X-linked recessive disorders much more frequently than females. A characteristic of X-linked inheritance is that fathers cannot pass X-linked traits to their sons. Nevertheless, if a phenomenon called lyonization occurs on 1 of the X chromosomes in females, some clinical manifestations of disease can be found in women as well as in men.70
The putative impact on retinoschisin protein structure by particular mutations was assessed in several research studies.71 Conservative RS1 missense mutations, which retain the same amino acid residue charge and similar size, are likely to alter protein structure only minimally; these are placed in the genetically “less severe” category. Other mutations are classified as putatively “more severe” because they cause major structural change or markedly reduce the production of retinoschisin protein, such as the addition or subtraction of a cysteine residue that would disrupt a disulfide bond important for tertiary folding configuration. Some frameshift mutations would alter all downstream amino acid residues; splice site alterations would disrupt coding at the intron-exon junction; nonsense mutations would be likely to cause premature protein truncation cause rapid degradation of retinoschisin protein, as shown by biochemical analysis.
Pathophysiology
Retinoschisin is expressed most abundantly in photoreceptor inner segments.72,73 It is secreted as a disulphide-linked octamer that helps maintain the structural integrity of the retina.74 The loss of functional retinoschisin leads to formation of cystic spaces between the outer plexiform layer (OPL) and outer nuclear layer (ONL) of the retina.75,76 This manifests as a “spoke-wheel” pattern in the macula on ophthalmoscopy (Fig. 4). These anatomical changes are associated with progressively worsening visual acuity.77 In a mouse model, the photoreceptors begin to degenerate at an early age and increasingly disrupt the OPL, where the photoreceptors synapse on second-order bipolar cells of the inner nuclear layer (INL). After a majority of the photoreceptors have succumbed, the rate of INL disruption decreases.78
History
The natural history of XLRS has been described as progressive. Typical presentation is complaints of poor vision in childhood. Occasionally patients present earlier during infancy with nystagmus, strabismus, high hyperopia, foveal schisis, retinal hemorrhage, or retinal detachment. There is a wide variability in disease severity, ranging from reduced visual acuity, 20/60 to 20/120 range to legal blindness, even among patients carrying the same mutation. The disease can also present as first diagnosis with vitreous hemorrhage or retinal detachment. These complications occur commonly and are the main causes of complete vision loss over the course of life. Family history is consistent with X-linked inheritance (affected maternal grandfather and carrier mother).
Signs and Symptoms
Vision loss is the most common symptom. Strabismus, nystagmus, amblyopia, high refractive errors, and visual field defects are also found among boys with XLRS.
Physical examination
Ophthalmoscopy represents the major tool for diagnosis, although at very young age the fundi may appear normal. An examination including the posterior pole is recommended to identify macular lesions, such as macular cavities or schisis, also signs associated with schisis such as vitreous veils. (Fig. 4) Vitreous veils have been considered indicators of high risk for retinal detachment.
Imaging, ancillary studies and diagnosis
Electroretinographic (ERG) responses indicate dysfunction at the outer plexiform layer (OPL). The contribution of bipolar cells, manifested in the cornea-positive b-wave, is disproportionately reduced relative to the photoreceptor contribution, manifested in the cornea-negative a-wave (Fig. 4D). The resultant “negative ERG”69 is consistent with attenuation of the retinal signal as it crosses the OPL.79 However, more recent studies have shown photoreceptor involvement in XLRS.80,81 The adaptive optics (AO) retinal imager82 has been recognized as an additional diagnostic tool to detect OPL abnormalities in boys with XLRS.83 Optical coherence tomography (OCT) imaging demonstrates the typical intraretinal cystic spaces between the OPL and outer nuclear layer ONL of the retina (Fig. 4B).
Differential diagnosis should include Incomplete congenital stationary night blindness (CSNB), which has a similar electronegative waveform on ERG testing and reduced rod responses. However, patients with mutations in 1 of the CSNB genes are typically characterized by a more stable course of the symptoms over time.
The presence of macular schisis on the indirect ophthalmoscopy and OCT must be compared with other forms of schisis, such as that associated with high myopia, or with cystoid macular edema that may accompany a variety of diseases such as uveitis, retinal vein occlusion, or diabetes.
Therapeutic strategies
There are no effective treatments for XLRS. Gene replacement is the logical therapy, and worldwide there are high expectations for that. Gene therapy was successful in a mouse model.73 In the RS1-/y knockout mouse, intravitreal injection of an adeno-associated virus (AAV) carrying RS1 substantially slowed the rate of retinal degeneration and improved ERG function.73,78,84,85 However, in a phase I/IIa clinical trial,86 AAV8-RS1 gene therapy produced considerable inflammatory responses and only transient closure of cystic spaces in one patient. Why a therapy that was so effective in mice was so ineffective in humans has not been explained. A knockout differs from the common mutations causing human disease, in that the latter seldom results in no protein being translated,87 but rather translation of mutant protein.
Our recent ERG81,88,89 and AO in vivo cellular imaging83 observations in boys shifts emphasis for a therapeutic approach to the photoreceptors. The phototransduction cascade, like the similar cascade in bipolar cells, can be targeted by numerous classes of compounds. Some of these compounds may be of therapeutic value.
Carbonic anhydrase inhibitors (CAIs), for reasons not well understood, do help the XLRS retina.90 Multiple beneficial effects, including decreased central retinal thickness, improved visual acuity, and enhanced macular function (measured by both psychophysics and ERG), are documented following CAI therapy.91 CAIs are either instilled onto the eye (eg, dorzolamide) or systemically administered (eg, acetazolamide). They do not directly treat XLRS disease processes, but rather inhibit the catalysis of carbon dioxide and water to carbonic acid (which is, in turn, converted to bicarbonate). The putative mechanism of CAI therapy is transporting fluids out of the retina, reducing the volume of the cystic spaces,92,93 and perhaps promoting cellular adhesion.94 This is likely achieved by increasing acidification of the subretinal space. This thereby decreases the standing potential and increases fluid transport across the retinal pigment epithelium to the choroid.95,96
General management and prognosis
Patients with XLRS should be closely followed up by a retinal specialist or a pediatric ophthalmologist partnered with a retinal surgeon. Patients with XLRS should be advised to avoid contact sports. Those with high refractive errors are considered at higher risk for complications, such as retinal hemorrhage and retinal detachment. Those with low acuity may also benefit from low-vision aids, including electronic, computer-based, and optical aids. Orientation and mobility training is available through community resources.
Visual acuity often declines in childhood and adolescence but then stabilizes during adulthood—until a significant decline typically occurs in a patient’s fifties or sixties. Sometimes, severe complications develop, such as splitting of the retinal layers, retinal detachment, or vitreous hemorrhage. These eye abnormalities can further impair vision or cause blindness.
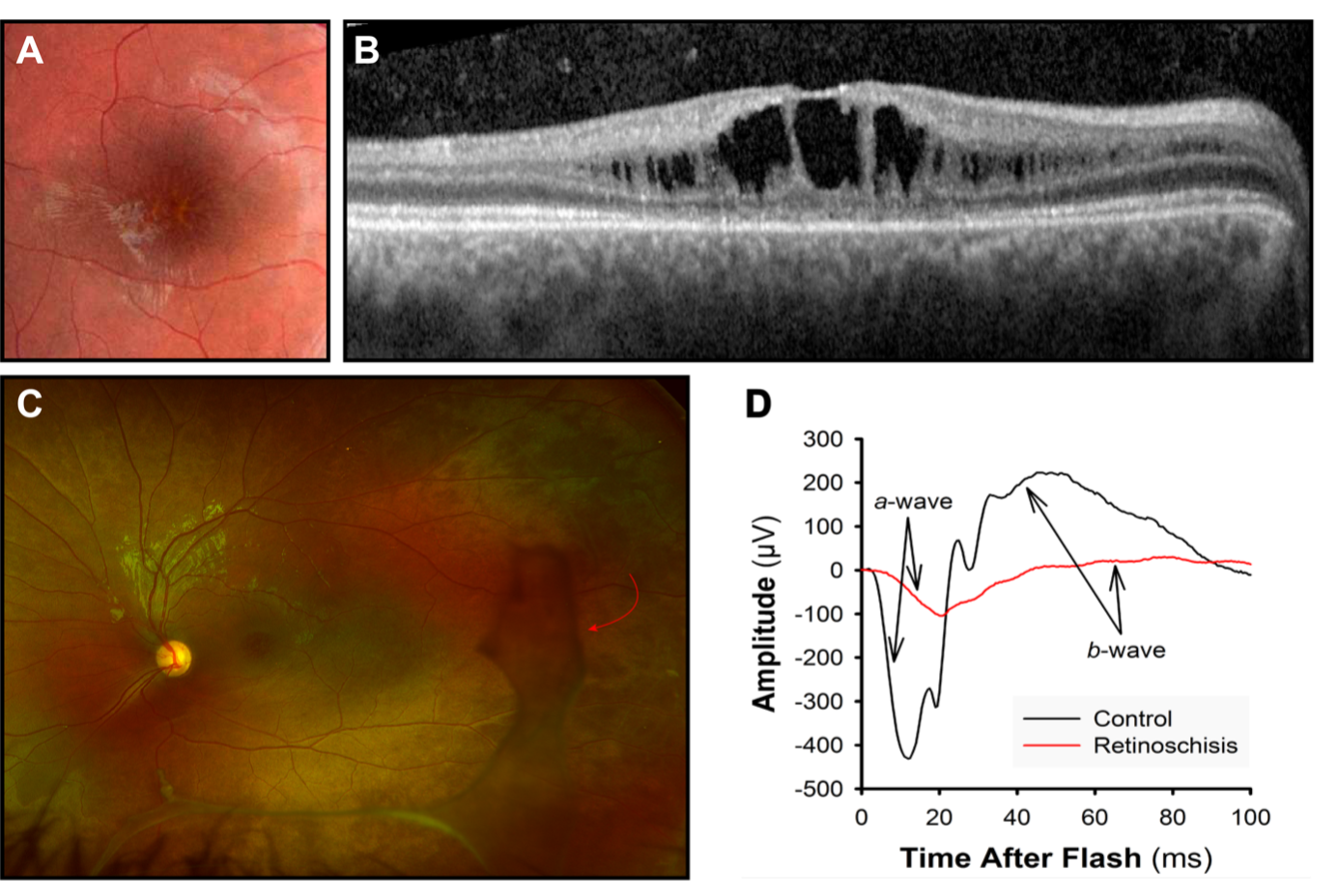
Fig 4. X-linked Retinoschisis. Retinoschisis appears as (A) a “spoke-wheel” pattern on fundus photographs of the macula and (B) dark spaces in optical coherence tomograms. (C) Ultra-widefield retinal image. In the peripheral retina, are visible vitreous veils (red arrow). (D) Both the negative-going a-wave that originates in photoreceptors and the positive-going b-wave that originates in photoreceptors are attenuated in retinoschisis.
Herein we have reviewed the most common disorders affecting pediatric macula, all associated with acuity deficits demanding supportive care. All together, these disorders are equally as common as amblyopia.75 They must be included in the differential diagnosis of visual impairment in childhood.
References
-
Lambertus S, Van Huet RAC, Bax NM, Hoefsloot LH, Cremers FPM, Boon CJF, Klevering BJ, Hoyng CB. Early-onset stargardt disease: Phenotypic and genotypic characteristics. Ophthalmology, 2015;122(2), 335–344. https://doi.org/10.1016/j.ophtha.2014.08.032.
-
Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30(2), 63–68. https://doi.org/10.1080/13816810802695550.
-
Palejwala NV, Gale MJ, Clark RF, Schlechter C, Weleber RG, Pennesi ME. Dystrophy Through Multimodality Diagnostic Imaging. 2003;1–12.
-
Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: Clinical features, molecular genetics, animal models and therapeutic options. Br Ophthalmol. 2017:101(1), 25–30. https://doi.org/10.1136/bjophthalmol-2016-308823.
-
Koenekoop RK The gene for Stargardt disease, ABCA4, is a major retinal gene: a mini-review. Ophthalmic Genet. 2003:24(2), 75–80. https://doi.org/10.1076/opge.24.2.75.13996.
-
Dandona L, Dandona R. Revision of visual impairment definitions in the International Statistical Classification of Diseases. BMC Med. 2006;4, 7. https://doi.org/10.1186/1741-7015-4-7.
-
Schönbach EM, Ibrahim MA, Strauss RW, Birch DG, Cideciyan AV, Hahn GA, Ho A, Kong X, Nasser F, Sunness JS, Zrenner E, Sadda SVR, West SK, Scholl HPN; Progression of Stargardt Disease Study Group. Fixation Location and Stability Using the MP-1 Microperimeter in Stargardt Disease: ProgStar Report No. 3. Ophthalmol Retina. 2017;1(1), 68–76. https://doi.org/10.1016/j.oret.2016.08.009.
-
Cukras CA, Wong WT, Caruso R, Cunningham D, Zein W, Sieving PA. Centrifugal expansion of fundus autofluorescence patterns in Stargardt disease over time. Arch Ophthalmol. (Chicago, Ill:1960), (2012):130(2), 171–179. https://doi.org/10.1001/archophthalmol.2011.332
-
Fishman GA. Fundus flavimaculatus. A clinical classification. Arch Ophthalmol. 1976;94(12):2061-2067.
-
Oh KT, Weleber RG, Stone EM, Oh DM, Rosenow J, Billingslea AM. Electroretinographic findings in patients with Stargardt disease and fundus flavimaculatus. Retina. (2004);24(6), 920–928. https://doi.org/10.1097/00006982-200412000-00013.
-
Lois N, Holder GE, Bunce C, Fitzke FW, Bird AC. Phenotypic subtypes of Stargardt macular: Dystrophy-fundus flavimaculatus. Arch Ophthal. 2001;119(3), 359–369. https://doi.org/10.1001/archopht.119.3.359
-
Kubota R, Birch DG, Gregory JK, Koester JM. Randomised study evaluating the pharmacodynamics of emixustat hydrochloride in subjects with macular atrophy secondary to Stargardt disease. Br J Ophthalmol. 2020;bjophthalmol-2020-317712. Advance online publication. https://doi.org/10.1136/bjophthalmol-2020-317712.
-
Johnson AA, Guziewicz KE, Lee CJ, Kalathur RC, Pulido JS, Marmorstein LY, Marmorstein AD. Bestrophin 1 and retinal disease. Prog Retin Eye Res. 2017; 58:45–69. https://doi.org/10.1016/j.preteyeres.2017.01.006.
-
Budiene B, Liutkeviciene R, Zaliuniene D. Best vitelliform macular dystrophy: literature review. Cent Eur J Med. 2014:9(6), 784–795. https://doi.org/10.2478/s11536-013-0333-8
-
Bitner H, Schatz P, Mizrahi-Meissonnier L, Sharon D, Rosenberg T. Frequency, genotype, and clinical spectrum of best vitelliform macular dystrophy: Data from a national center in Denmark. Am J Ophthalmol. 2012;154(2), 403-412. e4. https://doi.org/10.1016/j.ajo.2012.02.036.
-
Dalvin LA, Pulido JS, Marmorstein AD. Vitelliform dystrophies: Prevalence in Olmsted County, Minnesota, United States. Ophthalmic Genet. 2017:38(2), 143–147. https://doi.org/10.1080/13816810.2016.1175645
-
Frennesson CI, Wadelius C, Nilsson SEG. Best vitelliform macular dystrophy in a Swedish family: genetic analysis and a seven‐year follow‐up of photodynamic treatment of a young boy with choroidal neovascularization. Acta Ophthalmol. 2014; 92:238-242. https://doi.org/10.1111/aos.12142
-
Boon CJF, Theelen T, Hoefsloot EH, Van Schooneveld MJ, Keunen JEE, Cremers FPM, Klevering BJ, Hoyng CB. Clinical and molecular genetic analysis of best vitelliform macular dystrophy. Retina. 2009; 29(6):835–847. https://doi.org/10.1097/IAE.0b013e31819d4fda
-
Boon CJF, Klevering BJ, Leroy BP, Hoyng CB, Keunen JEE, den Hollander AI. Den. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 2009; 28(3), 187–205. https://doi.org/10.1016/j.preteyeres.2009.04.002
-
Gao, T., Tian, C., Hu, Q., Liu, Z., Zou, J., Huang, L., & Zhao, M. (2018). Clinical and Mutation Analysis of Patients with Best Vitelliform Macular Dystrophy or Autosomal Recessive Bestrophinopathy in Chinese Population. Biomed Res Int.2018; 4582816. https://doi.org/10.1155/2018/4582816
-
Mullins RF, Kuehn MH, Faidley EA, Syed NA, Stone EM. Differential Macular and Peripheral Expression of Bestrophin in Human Eyes and Its Implication for Best Disease. Invest Ophthalmol Vis Sci. 2007; 48(7)3372–3380. https://doi.org/10.1167/iovs.06-0868
-
Yardley J, Leroy BP, Hart-Holden N, et al. Mutations of VMD2splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC). Invest Ophthalmol Vis Sci. 2004;45(10):3683-3689. doi: https://doi.org/10.1167/iovs.04-0550.
-
Michaelides M, Urquhart J, Holder GE, et al. Evidence of genetic heterogeneity in MRCS (microcornea, rod-cone dystrophy, cataract, and posterior staphyloma) syndrome. Am J Ophthalmol. 2006;141(2), 418–420. https://doi.org/10.1016/j.ajo.2005.09.018
-
MacDonald IM, Lee T, Lawrence J. Bestrophinopathies. 2003 Sep 30 [Updated 2020 Jul 16]. In: Adam MP, Ardinger HH, Pagon RA, et al, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1167/
-
Apushkin MA, Fishman GA, Taylor CM, Stone EM. Novel de novo mutation in a patient with Best macular dystrophy. Arch Ophthalmol.2006;124(6):887–889. doi:10.1001/archopht.124.6.887
-
Fishman GA, Baca W, Alexander KR, Derlacki DJ, Glenn AM, Viana M. Visual acuity in patients with Best vitelliform macular dystrophy. Ophthalmology, 1993;100(11), 1665–1670. https://doi.org/10.1016/S0161-6420(93)31420-X
-
Querques G, Atmani K, Bouzitou-Mfoumou R, Leveziel N, Massamba N, Souied EH. Preferential hyperacuity perimeter in best vitelliform macular dystrophy. Retina (Philadelphia, Pa.), 2011; 31(5), 959–966. https://doi.org/10.1097/IAE.0b013e3181f441c1
-
Wittström E, Ponjavic V, Bondeson M-L, Andréasson S. Anterior segment abnormalities and angle-closure glaucoma in a family with a mutation in the BEST1 gene and Best vitelliform macular dystrophy. Ophthalmic Genet, 2011; 32(4), 217–227. https://doi.org/10.3109/13816810.2011.567884
-
Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas, S. The Lancet. 2017; 390(10108), 2183–2193. https://doi.org/10.1016/S0140-6736(17)31469-1
-
Miller SA. Multifocal Best's vitelliform dystrophy. Arch Ophthalmol. 1977; Jun;95(6):984-90. doi: 10.1001/archopht.1977.04450060070004. PMID: 869757.
-
Querques G, Zerbib J, Santacroce R, et al. Functional and clinical data of Best vitelliform macular dystrophy patients with mutations in the BEST1 gene. Mol Vis.2009; 15:2960–2972.
-
de Souza C, Mello L, Gomez F, Morizot E. Best vitelliform macular dystrophy in a large Brazilian family. Int J Retina Vitreous. 2019; 5:6. https://doi.org/10.1186/s40942-019-0156-0
-
Ong SS, Patel TP, Singh MS. Optical coherence tomography angiography imaging in inherited retinal diseases. J Clinical Med. 2019; 8(12), 2078. https://doi.org/10.3390/jcm8122078
-
Guduru A, Gupta A, Tyagi M, Jalali S, Chhablani J. Optical coherence tomography angiography characterisation of Best disease and associated choroidal neovascularisation. 2018; 102(4):444–447. https://doi.org/10.1136/bjophthalmol-2017-310586
-
Leu J, Schrage NF, Degenring RF. Choroidal neovascularisation secondary to Best's disease in a 13-year-old boy treated by intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2007;245:1723–1725.
-
Andrade RE, Farah ME, Costa RA. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization in best disease. Am J Ophthalmol. 2003;136:1179–81.
-
Marano F, Deutman AF, Leys A, Aandekerk AL. Hereditary retinal dystrophies and choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2000;238:760–764.
-
Chaudhary KM, Mititelu M, Lieberman RM. An evidence-based review of vascular endothelial growth factor inhibition in pediatric retinal diseases: part 2. Coats' disease, Best disease, and uveitis with childhood neovascularization. J Pediatr Ophthalmol Strabismus, 2013; 50(1), 11–19. https://doi.org/10.3928/01913913-20120821-02
-
Tewari R, Kumar V, Ravani R, Dubey D, Chandra P, Kumar A. Macular hole-associated retinal detachment in Best vitelliform dystrophy, Indian J Ophthalmol. 2018; 66(5):708-711. doi: 10.4103/ijo.IJO_1046_17
-
Peart S, Ramsay A, Khan Q, A, Leong T, Gordon-Bennett P. Large, spontaneous macular hole with posterior pole detachment in a patient with Best vitelliform macular dystrophy. Case Rep Ophthalmol. 2019;10:221-226. doi: 10.1159/000501845
-
Nourinia R, Roshandel D, Lima BS, Sayanjali S. Best disease associated with macular hole, Retin Cases Brief Rep. 2015; 9(1):7-12. doi: 10.1097/ICB.0000000000000068
-
Tripathy K, Salini B. Best Disease. [Updated 2021 Feb 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537290/
-
Summers C G., Hand J L., (2021) Oculocutaneous albinism, in Levy M & Corona R. (Eds), Uptodate https://www.uptodate.com/contents/oculocutaneous-albinism. Retrieved on May 8, 2021.
-
Biswas S, Lloyd IC. Oculocutaneous albinism. Arch Dis Child. 1999; 80(6), 565 LP – 569. https://doi.org/10.1136/adc.80.6.565
-
Chong GT, Farsiu S, Freedman SF, et al. Abnormal foveal morphology in ocular albinism imaged with spectral-domain optical coherence tomography. Arch Ophthalmol.2009;127(1):37–44. doi:10.1001/archophthalmol.2008.550
-
McCafferty BK, Wilk MA, McAllister JT, et al. Clinical insights into foveal morphology in albinism. J Pediatr Ophthalmol Strabismus, 2015; 52(3):167–172. https://doi.org/10.3928/01913913-20150427-06
-
Simeonov DR, Wang X, Wang C. DNA variations in oculocutaneous albinism: an updated mutation list and current outstanding issues in molecular diagnostics. Hum Mutat.2013; 34(6), 827–835. https://doi.org/10.1002/humu.22315
-
Kamaraj B, Purohit R. Mutational analysis of oculocutaneous albinism: a compact review. BioMed Res Int. 2014;905472. https://doi.org/10.1155/2014/905472
-
Montoliu L, Grønskov K, Wei A‐H, et al. Increasing the complexity: new genes and new types of albinism. Pigment Cell Melanoma Res. 2014; 27:11-18. https://doi.org/10.1111/pcmr.12167
-
Grønskov K, Ek J, Brondum-Nielsen K. Oculocutaneous albinism. Orphanet J Rare Dis.2007;2, 43. https://doi.org/10.1186/1750-1172-2-43
-
Gao X, Liu T, Cheng, X, et al. A novel GPR143 mutation in a Chinese family with X‑linked ocular albinism type 1. Mol Med Rep. 2020; 21, 240-248. https://doi.org/10.3892/mmr.2019.10813
-
Jeffery G. The albino retina: an abnormality that provides insight into normal retinal development. Trends Neurosci. 1997; 20(4), 165–169. https://doi.org/10.1016/S0166-2236(96)10080-1
-
Hoffmann MB, Tolhurst DJ, Moore AT, Morland AB. Organization of the visual cortex in human albinism. J Neurosci. 2003; 23(26), 8921 LP – 8930. https://doi.org/10.1523/JNEUROSCI.23-26-08921.2003
-
Rebsam A, Bhansali P, Mason CA. Eye-specific projections of retinogeniculate axons are altered in albino mice. J Neurosci. 2012; 32(14), 4821–4826. https://doi.org/10.1523/JNEUROSCI.5050-11.2012
-
Bhansali P, Rayport I, Rebsam A, Mason C. Delayed neurogenesis leads to altered specification of ventrotemporal retinal ganglion cells in albino mice. Neural Dev.2014; 18:9, 11. https://doi.org/10.1186/1749-8104-9-11
-
Mason C, Guillery R. Conversations with Ray Guillery on albinism: linking Siamese cat visual pathway connectivity to mouse retinal development. Eur J Neurosci. 2019; 49:913-927. https://doi.org/10.1111/ejn.14396
-
McAllister JT, Dubis AM, Tait DM, et al. Arrested development: high-resolution imaging of foveal morphology in albinism. Vision Res. 2010; 50(8):810–817. https://doi.org/10.1016/j.visres.2010.02.003
-
Fulton AB, Albert DM, Craft JL. Human albinism. Light and electron microscopy study. Arch Ophthalmol. 1978; 96(2):305-310. doi: 10.1001/archopht.1978.03910050173014. PMID: 629678.
-
Akeo K, Shirai S, Okisaka S, at al. Histology of fetal eyes with oculocutaneous albinism. Arch Ophthalmol. 1996; 114(5):613-6. doi: 10.1001/archopht.1996.01100130605021. PMID: 8619776
-
Federico JR, Krishnamurthy K. Albinism. [Updated 2020 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519018/
-
Bertsch M, Floyd M, Kehoe T, Pfeifer W, Drack A V. The clinical evaluation of infantile nystagmus: What to do first and why. Ophthalmic Genet. 2017;38(1):22-33. doi:10.1080/13816810.2016.1266667.
-
PalB, Mohamed MD, Keen TJ, et al. A new phenotype of recessively inherited foveal hypoplasia and anterior segment dysgenesis maps to a locus on chromosome 16q23.2–24.2. J Med Genet. 2004;41:772-777.
-
Ehrenberg M, Bagdonite-Bejarano L, Fulton AB, Orenstein N, Yahalom C. Genetic causes of nystagmus, foveal hypoplasia and subnormal visual acuity- other than albinism. Ophthalmic Genet. 2021; 42(3):243-251. doi: 10.1080/13816810.2021.1888128. Epub 2021 Feb 17. PMID: 33594928
-
Wack MA, Peachey NS, Fishman GA, Electroretinographic findings in human oculocutaneous albinism. Ophthalmology. 1989; 96(12):1778-1785, ISSN 0161-6420,https://doi.org/10.1016/S0161-6420(89)32662-5.
-
Hu Z, Wang K, Bertsch M, et al. Correlation between electroretinography, foveal anatomy and visual acuity in albinism. Doc Ophthalmol. 2019; 139(1): 21–32. https://doi.org/10.1007/s10633-019-09692-9
-
Adams DR, Menezes S, Jauregui R, et al. One-year pilot study on the effects of nitisinone on melanin in patients with OCA-1B. JCI insight. 2019; 4(2):e124387. Advance online publication. https://doi.org/10.1172/jci.insight.124387
-
Summers CG, Connett JE, Holleschau AM, et al. Does levodopa improve vision in albinism? Results of a randomized, controlled clinical trial. Clinl Exp Ophthalmol. 2014; 42(8):713–721. https://doi.org/10.1111/ceo.12325
-
Nelwan M, Treat oculocutaneous albinism with gene therapy. Journal of Advances in Biology & Biotechnology. 2018; 16(3):1-12, 2017. DOI:10.9734/JABB/2017/38504, Available at SSRN: https://ssrn.com/abstract=2561722or http://dx.doi.org/10.2139/ssrn.2561722
-
George ND, Yates JR, Moore AT. X linked retinoschisis. Br J Ophthalmol. 1995; 79(7):697-702. doi:10.1136/bjo.79.7.697
-
Jay B. X-linked retinal disorders and the Lyon hypothesis. Trans Ophthalmol Soc U K. 1985; 104 (Pt 8): 836–844.
-
Bowles K, Cukras C, Turriff A, et al. X-linked retinoschisis: RS1 mutation severity and age affect the ERG phenotype in a cohort of 68 affected male subjects. Invest Ophthalmol Vis Sci, 2011; 52(12), 9250-9256. doi:10.1167/iovs.11-8115
-
Molday LL, Hicks D, Sauer CG, Weber BH, Molday RS. Expression of X-linked retinoschisis protein RS1 in photoreceptor and bipolar cells. Invest Ophthalmol Vis Sci. 2001; 42(3):816-825.
-
Ou J, Vijayasarathy C, Ziccardi L, et al. Synaptic pathology and therapeutic repair in adult retinoschisis mouse by AAV-RS1 transfer. J Clin Invest. 2015; 125(7):2891-2903. doi:10.1172/JCI81380
-
Wu WHW, Wong JP, Kast J, Molday RS. RS1, a discoidin domain-containing retinal cell adhesion protein associated with X-linked retinoschisis, exists as a novel disulfide-linked octamer. J Biol Chem, 2005; 280(11), 10721-10730. doi:10.1074/jbc.M413117200
-
Altschwager P, Ambrosio L, Swanson EA, Moskowitz A, Fulton AB. Juvenile macular degenerations. Semin Pediatr Neurol, 2017; 24(2):104-109. doi:10.1016/j.spen.2017.05.005
-
Gerth C, Zawadzki RJ, Werner JS, Héon E. Retinal morphological changes of patients with X-linked retinoschisis evaluated by Fourier-domain optical coherence tomography. Arch Ophthalmol, 2008; 126(6), 807-811. doi:10.1001/archopht.126.6.807
-
Eksandh LC, Ponjavic V, Ayyagari R, et al. Phenotypic expression of juvenile X-linked retinoschisis in Swedish families with different mutations in the XLRS1 gene. Arch Ophthalmol, 2000:118(8), 1098-1104. doi:10.1001/archopht.118.8.1098
-
Takada Y, Vijayasarathy C, Zeng Y, Kjellstrom S, Bush RA, Sieving PA. Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest Ophthalmol Vis Sci, 2008:49(8), 3677-3686. doi:10.1167/iovs.07-1071
-
Bradshaw K, George N, Moore A, Trump D. Mutations of the XLRS1 gene cause abnormalities of photoreceptor as well as inner retinal responses of the ERG. Doc Ophthalmol. 1999:98(2), 153-173. doi:10.1023/a:1002432919073
-
Khan NW, Jamison JA, Kemp JA, Sieving PA. Analysis of photoreceptor function and inner retinal activity in juvenile X-linked retinoschisis. Vision Res, 2001:41(28), 3931-3942. doi:10.1016/s0042-6989(01)00188-2
-
Ambrosio L, Hansen RM, Kimia R, Fulton AB. Retinal Function in X-Linked Juvenile Retinoschisis. Invest Ophthalmol Vis Sci, 2019; 60(14), 4872-4881. doi:10.1167/iovs.19-27897
-
Hammer DX, Ferguson RD, Mujat M, et al. Multimodal adaptive optics retinal imager: design and performance. J Opt Soc Am A Opt Image Sci Vis. 2012; 29(12), 2598-2607. doi:10.1364/JOSAA.29.002598
-
Ambrosio L, Williams JS, Gutierrez A, et al. Carbonic anhydrase inhibition in X-linked retinoschisis: An eye on the photoreceptors. Exp Eye Res. 2021; 202: doi:10.1016/j.exer.2020.108344
-
Molday LL, Min S-H, Seeliger MW, et al. Disease mechanisms and gene therapy in a mouse model for X-linked retinoschisis. Adv Exp Med Biol. 2006; 572, 283-289. doi:10.1007/0-387-32442-9_39
-
Weber BH, Schrewe H, Molday LL, et al. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci U S A, 2002; 99(9), 6222-6227. doi:10.1073/pnas.092528599
-
Cukras C, Wiley HE, Jeffrey BG, et al. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol Ther. 2018:26(9):2282-2294. doi:10.1016/j.ymthe.2018.05.025
-
Sergeev YV, Vitale S, Sieving PA, et al. Molecular modeling indicates distinct classes of missense variants with mild and severe XLRS phenotypes. Hum Mol Genet. 2013:22(23):4756-4767. doi:10.1093/hmg/ddt329
-
Akula JD, Ambrosio L, Howard FI, Hansen RM, Fulton AB. Extracting the ON and OFF contributions to the full-field photopic flash electroretinogram using summed growth curves. Exp Eye Res. 2019; 189: doi:10.1016/j.exer.2019.107827
-
Tanimoto N, Akula JD, Fulton AB, Weber BHF, Seeliger MW. Differentiation of murine models of "negative ERG" by single and repetitive light stimuli. Doc Ophthalmol. 2016; 132(2), 101-109. doi:10.1007/s10633-016-9534-1
-
Pennesi ME, Birch DG, Jayasundera KT; for the Xlrs-Study Group. Prospective evaluation of patients with X-linked retinoschisis during 18 months. Invest Ophthalmol Vis Sci. 2018; 59:5941–5956.
-
Stridh MH, Alt MD, Wittmann S, et al. Lactate flux in astrocytes is enhanced by a non-catalytic action of carbonic anhydrase II. J Physiol. 2012; 590;2333–2351.
-
Testa F, Di Iorio V, Gallo B, et al. Carbonic anhydrase inhibitors in patients with X-linked retinoschisis: effects on macular morphology and function. Ophthalmic Genet. 2019; 40:207–212.
-
Thobani A, Fishman GA. The use of carbonic anhydrase inhibitors in the retreatment of cystic macular lesions in retinitis pigmentosa and X-linked retinoschisis. Retina. 2011; 31, 312–315.
-
Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999; 97:387–397.
-
Marmor MF. Hypothesis concerning carbonic anhydrase treatment of cystoid macular edema: example with epiretinal membrane. Arch Ophthalmol. 1990; 108:1524–1525.
-
Verbakel SK, van de Ven JPH, Le Blanc LMP, et al. Carbonic anhydrase inhibitors for the treatment of cystic macular lesions in children with X-linked juvenile retinoschisis. Invest Ophthalmol Vis Sci. 2016; 57, 5143–5147.