EPIDEMIOLOGY
Global Information
The prevalence of congenital corneal opacities (CCO) is estimated to be 3 in100,000 newborns. This number increases to 6 in 100,000 if congenital glaucoma patients are included. Some retrospective studies have shown Peters anomaly to be the most common congenital corneal opacity presenting to a university cornea clinic.
Regional Information (ASIA-PACIFIC)
| Table 1. Percentage of Keratoplasties for Congenital Corneal Opacities |
| Study |
Country |
Percentage |
| Dada et al, 1999 |
India |
12.28% |
| Shi et al, 2007 |
China |
12.9% |
| Chang et al, 2010 |
Korea |
5.5% |
- A study of the indications for keratoplasty in New Zealand reported that 16% of the cases were for congenital corneal disorders (Patel et al, 2005).
DIFFERENTIAL DIAGNOSIS
- Corneal dystrophies:
- Congenital hereditary endothelial dystrophy
- Congenital hereditary stromal dystrophy
- Posterior polymorphous dystrophy
- Posterior amorphous corneal dystrophy
- Anterior segment dysgenesis (Figure 1), sclerocornea, congenital anterior staphyloma)
- Corneal dermoids
- Infections (congenital rubella, herpes simplex, bacterial)
- Trauma (forceps delivery trauma)
- Congenital glaucoma
- Metabolic disorders (mucopolysaccharidoses, mucolipidoses)
- Posterior corneal defects (posterior keratoconus)
- Whole globe abnormalities (microphthalmia)
- Developmental anomalies of iridotrabecular system or lens (Axenfeld-Rieger syndrome, Figure 2)
- Table 2 highlights the differential diagnosis of infantile corneal opacities and the method of diagnosis.
| Table 2. Differential Diagnosis of Infantile Corneal Opacities |
| Entity |
Location and Description of Opacity |
Other Signs |
Method of Diagnosis |
| Sclerocornea (total corneal opacification) |
Peripheral opacity, clearest centrally; unilateral or bilateral often vascularized |
Flat cornea |
Inspection; anterior segment imaging |
| Forceps injury |
Central opacity; unilateral |
Breaks in Descemet membrane |
History |
| Posterior corneal defect |
Central opacity; unilateral or bilateral |
Iris adherence to cornea; posterior keratoconus |
Inspection |
| Infection |
Central or diffuse; unilateral or bilateral |
Dendrites, infiltrate, ulceration |
Culture; PCR, serologic tests |
| Mucopolysaccharidosis, mucolipidosis |
Diffuse opacity; bilatera |
Smooth epithelium |
Conjunctival biopsy; biochemical testing |
| Congenital hereditary stromal dystrophy (CHSD) |
Diffuse opacity; bilateral |
Stromal opacities; normal thickness; normal epithelium |
Examination of family members for autosomal dominance |
| Congenital hereditary endothelial dystrophy (CHED) |
Diffuse opacity; bilateral |
Thickened cornea |
Inspection |
| Infantile glaucoma |
Diffuse opacity; unilateral or bilateral |
Enlarged cornea; breaks in Descemet membrane |
tonometry for elevated intraocular pressure |
| Dermoid |
Inferotemporal opacity; unilateral; elevated; surface hair; keratinized; usually limbal |
Associated with Goldenhar syndrome |
Inspection |
Reproduced, with permission, from Raab EL, Basic and Clinical Science Course, Section 6. Pediatric Ophthalmology and Strabismus. American Academy of Ophthalmology, 2014–2015.
PATHOPHYSIOLOGY / DEFINITION
The pathophysiology depends on the specific entity (Chart 1).
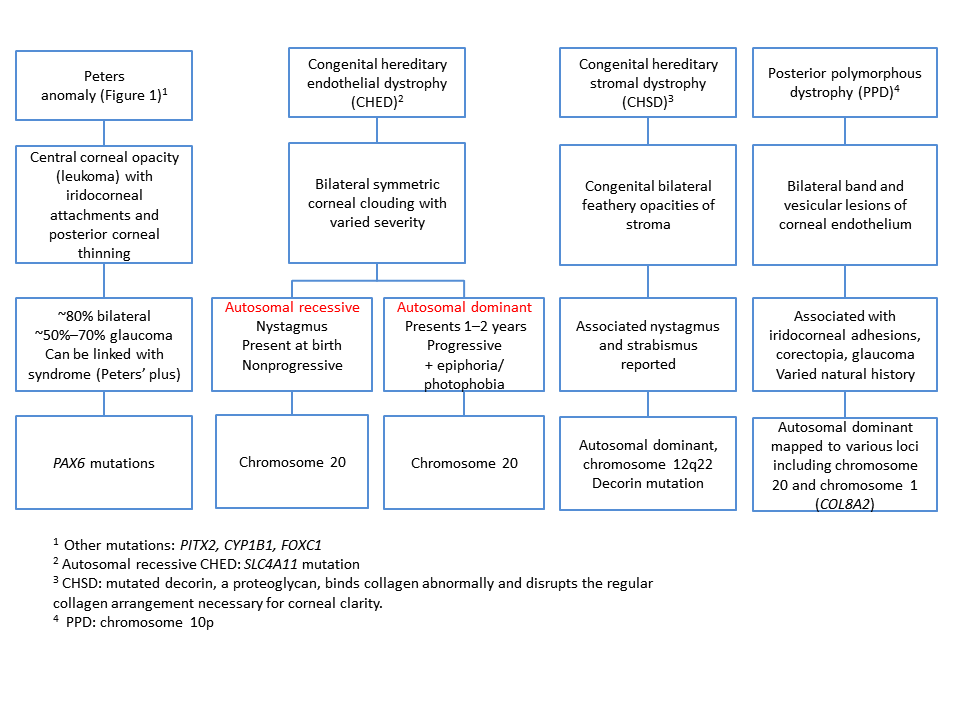
Chart 1. Differential diagnosis for congenital opacities, Part 1. See the Image Library for figures.
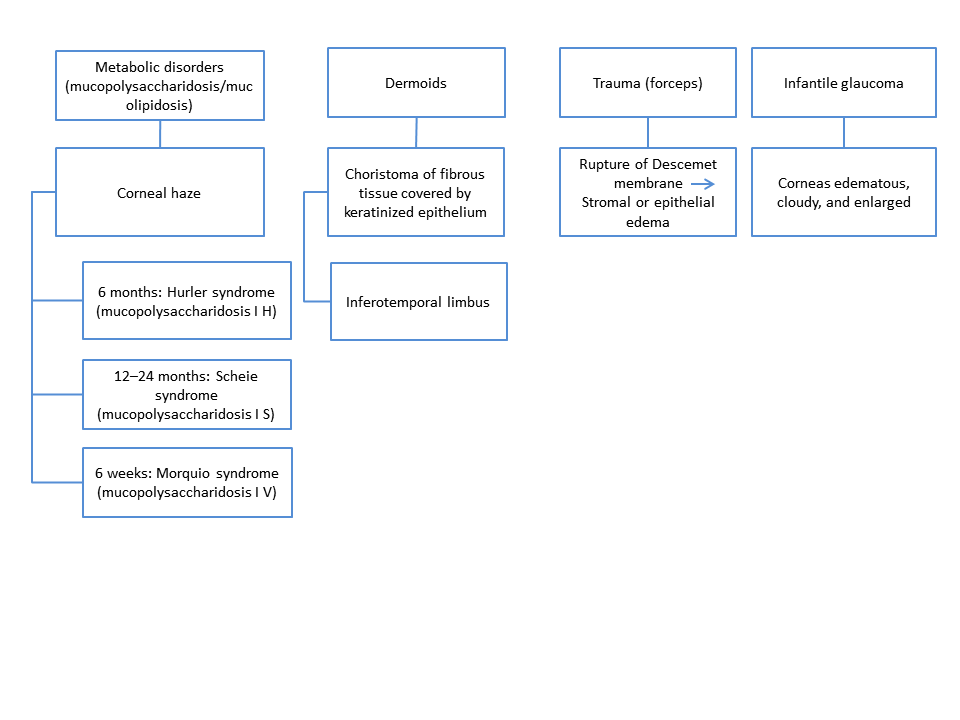
Chart 1. Differential diagnosis for congenital opacities, Part 2.
Signs, Symptoms
Signs
See Table 3 for signs and associated conditions.
| Table 3. Congenital Corneal Opacities: Signs and Conditions |
| Sign |
Associated Condition |
| Central opacity |
Peters anomaly (Figure 3)
Forceps injury
Posterior corneal defects |
| Peripheral opacity |
Sclerocornea
Dermoid |
| Diffuse opacity |
Infantile glaucoma
Congenital hereditary stromal dystrophy
Congenital hereditary endothelial dystrophy
Mucopolysaccharidoses/mucolipidoses |
| Bilateral clouding |
Congenital hereditary endothelial dystrophy
Congenital hereditary stromal dystrophy
Posterior polymorphous dystrophy
Mucopolysaccharidoses/mucolipidoses |
| Unilateral clouding |
Forceps injury
Dermoid |
| Bilateral or unilateral clouding |
Sclerocornea
Posterior corneal defects
Infantile glaucoma |
- Congenital hereditary endothelial dystrophy
- Autosomal dominant: irregularly thickened Descemet membrane with multiple disorganized fibrillar deposits in the posterior nonbanded layer and endothelial degeneration
- Autosomal recessive: evenly thickened Descemet membrane
- Congenital hereditary stromal dystrophy
- Abnormal stromal fibers with disorders lamellae
- Irregular Bowman membrane posteriorly
- Posterior polymorphous dystrophy
- Band-like lesions on corneal endothelium (Figure 4)
- Iridocorneal adhesions
- Corectopia (Figure 5)
- Glaucoma
- Endothelium is stratified and endothelial cells resemble epithelial cells with microvilli and desmosomes (Figure 6)
- Associated ocular conditions of Peters anomaly:
- Posterior corneal thinning involving the posterior stroma, Descemet membrane and endothelium
- Glaucoma (50%–70% of cases)
- Anterior staphyloma
- Coloboma (Figure 7)
- Sclerocornea (Figure 8)
- Microphthalmia (Figure 9)
- Aniridia (Figure 10)
- Dysgenesis of the angles structures
- Peters plus syndrome
- Short stature
- Mental retardation
- Abnormal ears
- Brachymorphy
- Cleft lip and palate
- Dermoid (Figure 11)
- Goldenhar syndrome (incomplete development of ear, nose, soft palate, lip, and mandible, usually unilaterally; can also present with organ dysgenesis and scoliosis)
- Sclerocornea (see Figure 8)
- Forceps trauma
- Infantile glaucoma
- Enlarged cornea
- Breaks in Descemet membrane
- Mucopolysaccharidoses/mucolipidoses (Figure 13)
- Posterior corneal defects
- Iris adherence to cornea
- Posterior keratoconus (Figure 14)
Symptoms
- Poor visual acuity
- Congenital hereditary endothelial dystrophy
- Autosomal recessive: nystagmus
- Autosomal dominant: epiphora and photophobia
- Congenital hereditary stromal dystrophy
- Posterior polymorphous dystrophy
- Usually asymptomatic
MANAGEMENT
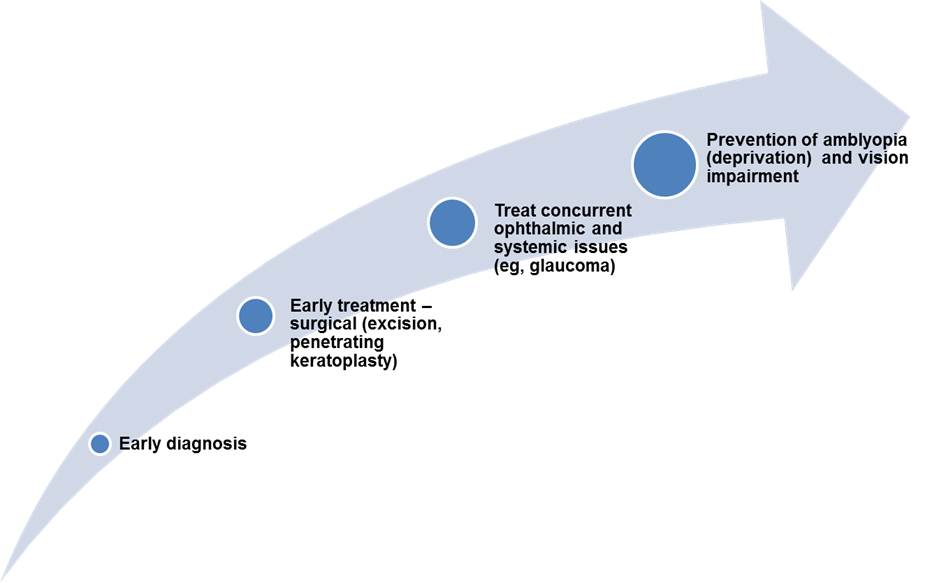
Chart 2. Management of of congenital corneal opacities.
Global Information
- Treatment of congenital corneal opacities is generally surgical and depends on the entity.
- If the opacity is dense and bilateral, keratoplasty for one eye should be considered as soon as possible to prevent amblyopia.
- If the opacity is unilateral, the decision to treat is more difficult.
- In resource-limited eye units, the main treatment option may be to do an optical iridectomy so that some vision can be stimulated through the clear area of the cornea.
- Unfortunately, treatment is often complicated and outcomes are visually unrewarding.
- This is due to several factors: penetrating keratoplasty in infants is technically demanding due to the reduced scleral rigidity and increased elasticity that allows anterior displacement of the lens-iris diaphragm and wound retraction, making suturing more difficult. There is also a risk of spontaneous lens expulsion.
- Postoperative complications associated with this surgery are refractive errors and persistently severe amblyopia.
- Higher rates of graft failure are associated with severe disease, larger donor cornea size, coexisting central nervous abnormalities, and anterior synechiae.
- Preoperative predictors of graft failure include stromal vessels, total limbal opacification, and preoperative glaucoma.
- In general, higher surgical success rates are reported in cases of posterior polymorphous dystrophy and congenital hereditary endothelial dystrophy than in cases of Peters anomaly, sclerocornea, and congenital glaucoma.
- Dermoids may be removed by shaving or lamellar keratoplasty, but for deeper, more extensive dermoids, penetrating keratoplasty may be necessary.
CASE STUDY
History of Present Illness
A 30-day old male infant presented with near-total blindness and coarse irregular nystagmoid eye movements. No other signs of systemic disease were present. He was the first-born son of nonconsanguineous parents, and the family history was unremarkable.
Examination
Physical exam was normal except for gross clouding of both corneas. Visualization was marked reduced by the corneal opacities. No abnormalities were noted on ocular ultrasonography. The axial lengths of the right and left eyes were 19.31 mm and 19.18 mm respectively. The intraocular pressure (IOP) was 18 mmHg. Pachymetry revealed corneal thickness of approximately 950 mm in both eyes. Metabolic and laboratory tests were negative.
Treatment
Penetrating keratoplasty was performed on the right eye when the patient was 3 months old and on the left eye when the patient was 5 months old. A corneal flap of 7.75 mm was used in both cases. The graft for the right eye came from a 3-year-old donor and the graft for the left eye came from a 17-year-old donor. They were prepared with Hessburg Barron vacuum trephine with a diameter of 7.5 mm and sutured with double running sutures. The sutures were removed after 6 months. Postoperative care included dexamethasone corticosteroid, and netilmicin drops 4 times a day for the first month and fluorometholone 0.1% drops 2 times a day for the next 45 days.
Follow-Up
A few days postoperatively, the infant began to show interest in and follow objects and luminous targets. Forty days after surgery, the implanted cornea was transparent; the axial length of the right eye was 21.69 mm and the IOP was 14 mmHg. At 9 months, the nystagmus diminished considerably and the baby was showing clear interest in his surroundings. Examination under general anesthesia showed that both grafts were transparent. The axial lengths had increase to 23.05 mm on the right and 22.04 on the left. The IOP was 12 mmHg in both eyes. Corneal thickness was 550 mm in the right eye and 520 mm in the left eye. At 12 months of age, the anteroposterior axis had further increased in both eyes, and the infant began wearing glasses for optical correction.
Histology of Explanted Corneas
The endothelium of the explanted tissues was thickened, with severely altered cytotype and interdigitations between adjacent cells. Descemet membrane was thin and appeared completely amorphous. The keratocytes were arranged in uninterrupted rows with interdigitations between one cell and the next. The stromal fibrils lay parallel and were normal in caliber, but the interfibrillar space was reduced by half. These findings are consistent with a diagnosis of combined dystrophy of the endothelial cell with secondary involvement of Descemet membrane.
IMAGE LIBRARY
Pathophysiology/Definition
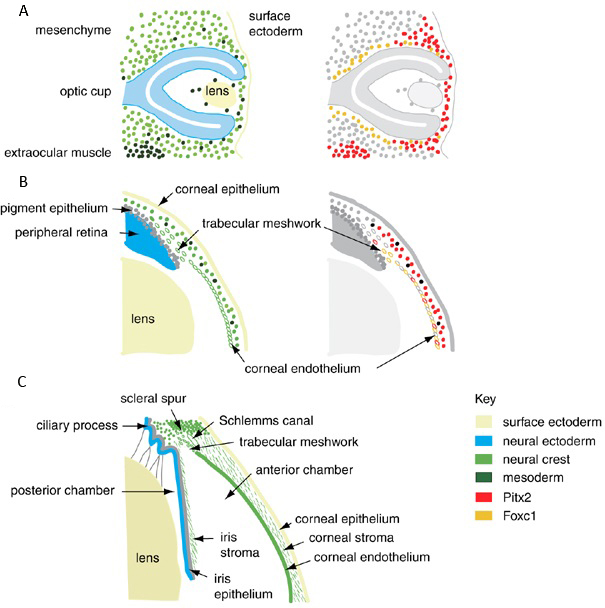
Figure 1. The embryonic and fetal development of the anterior segment of the eye. A. Optic cup stage. B. Formation of anterior chamber. C. Mature anterior segment depicting the lens, iris, irido-corneal angle, and the cornea. The color coding represents the embryonic origin of the anterior segment tissues and the pattern of expression of the Foxc1 and Pitx2, based on published expression data. Mutations in Foxc1 and Pitx2 disrupt the formation of the anterior segment in Peters anomaly. (Reproduced, with permission, from Sowden JC. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye (Lond). 2007;21(10):1310–8.)
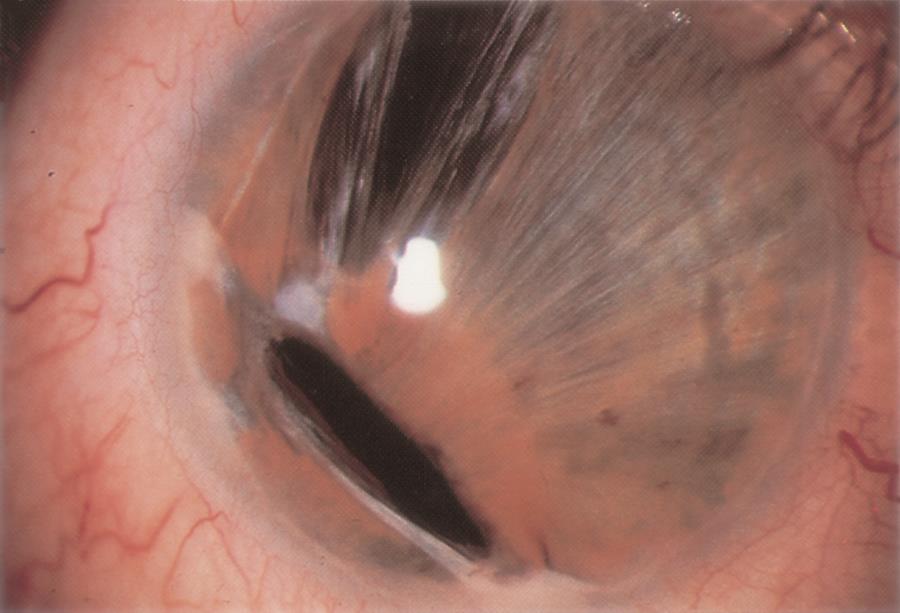
Figure 2. Sturge-Weber syndrome. A. Sturge-Weber syndrome with increased tortuosity of limbal blood vessels due to elevated venous pressure leading also to an increase in intraocular pressure. B. Sturge-Weber syndrome classically comprises the triad of port-wine facial telangectasias (nevus flammeus) in the distribution of the trigeminal nerve that respects the vertical midline, ipsilateral glaucoma, and intracranial angiomata. (© 2015 American Academy of Ophthalmology, www.aao.org).
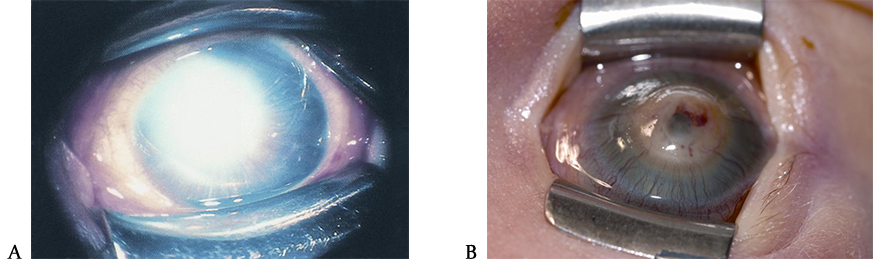
Figure 3. Peters anomaly. A. Central dense corneal opacity typical of Peters anomaly. B. Congenital anterior staphyloma associated with Peters anomaly. (© 2015 American Academy of Ophthalmology, www.aao.org.)
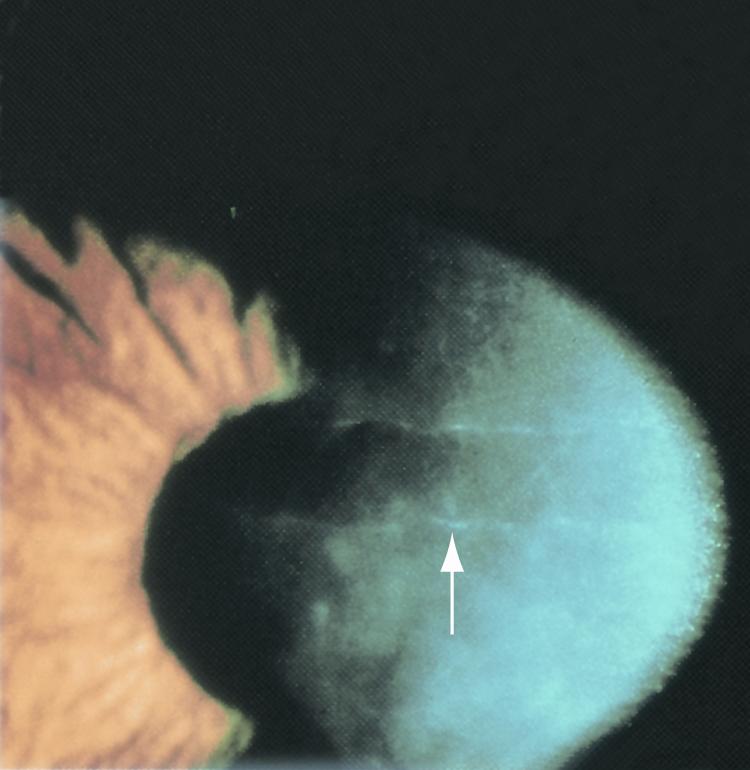
Figure 4. Band-like lesions on corneal endothelium (arrow) in eyes with posterior polymorphous dystrophy. (© 2015 American Academy of Ophthalmology, www.aao.org.)
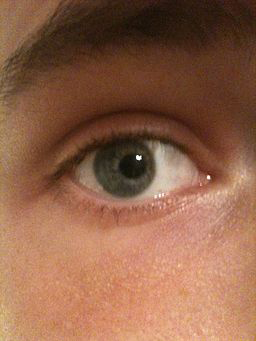
Figure 5. Corectopia. An eye with an off-centered pupil. (Courtesy Wachamalamanon/CC-BY-SA3.)
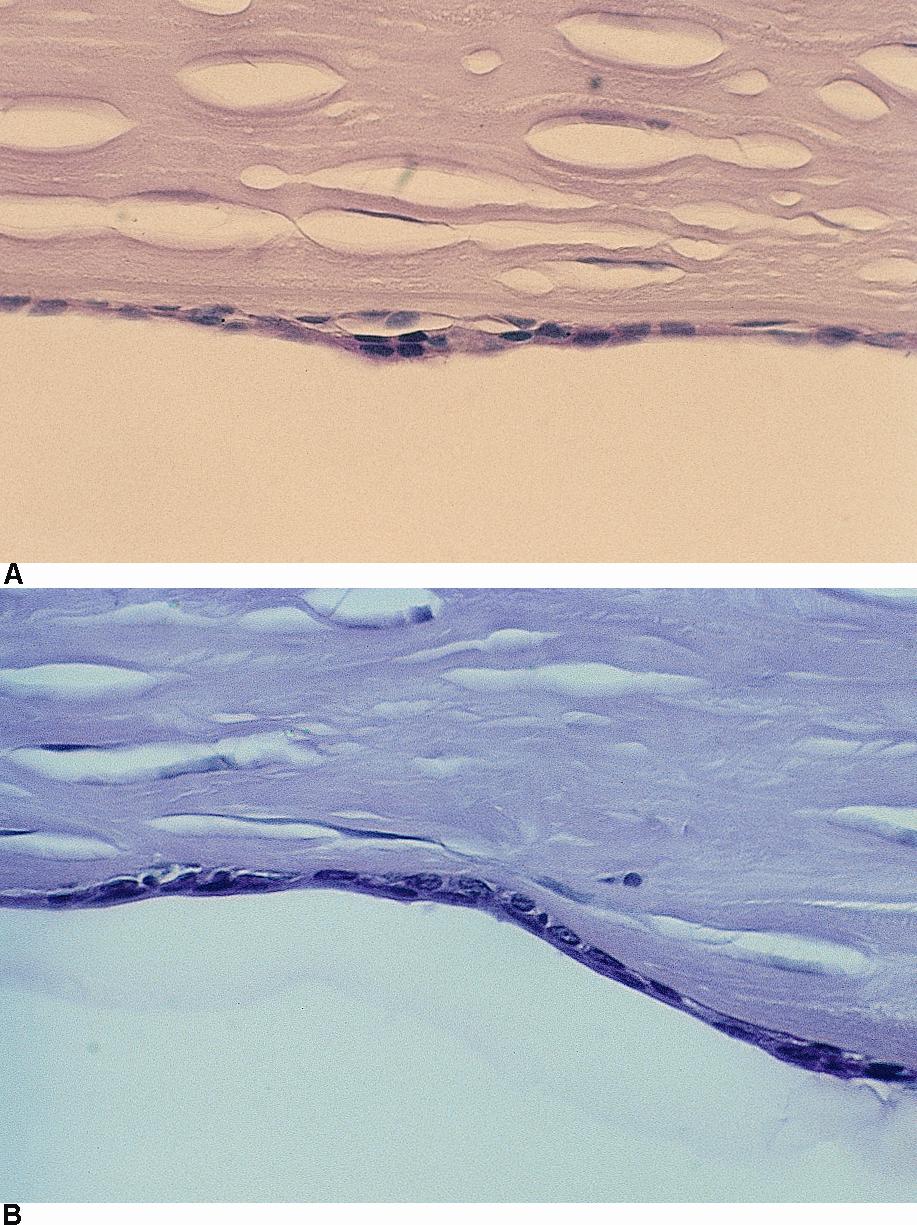
Figure 6. Posterior polymorphous dystrophy. Light microscopy showing (A) multilayered epithelialized endothelial cells and (B) epithelialization of endothelium. (© 2015 American Academy of Ophthalmology, www.aao.org.)
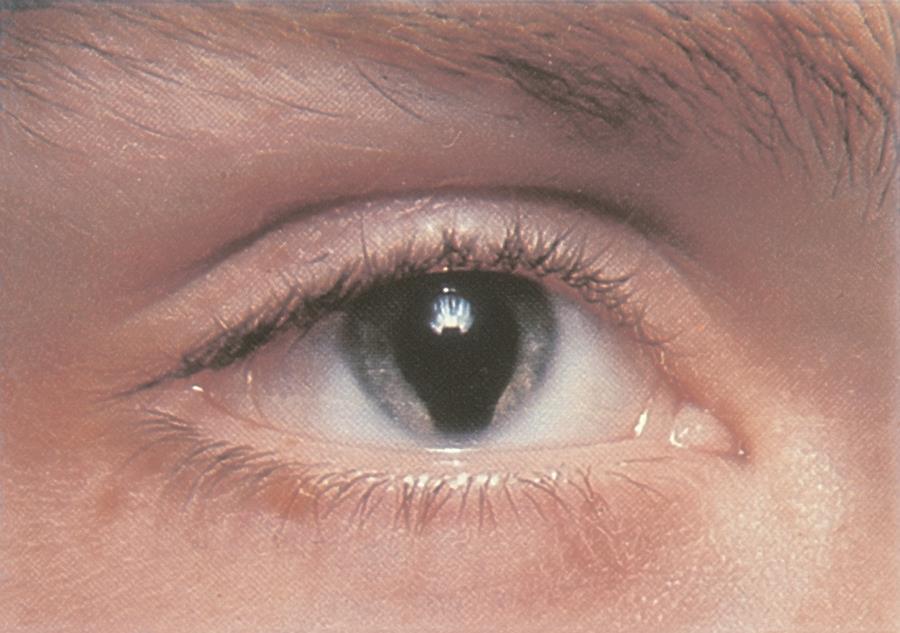
Figure 7. Iris coloboma in Peters anomaly. (© 2015 American Academy of Ophthalmology, www.aao.org.)
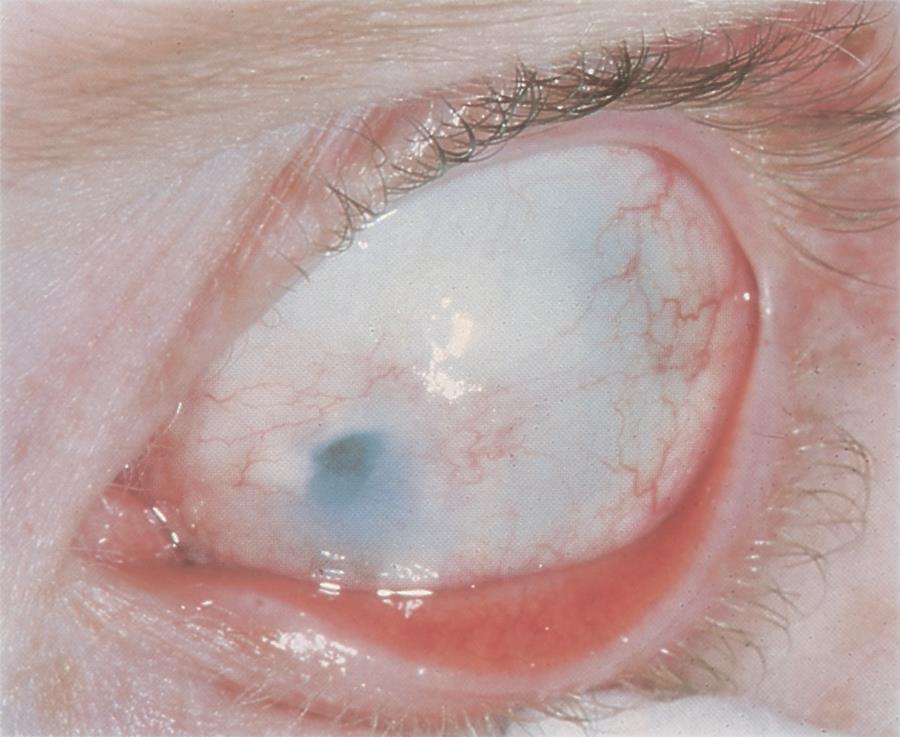
Figure 8. Scleral-like clouding of the cornea and corneal flattening in a child with sclerocornea. (© 2015 American Academy of Ophthalmology, www.aao.org.)
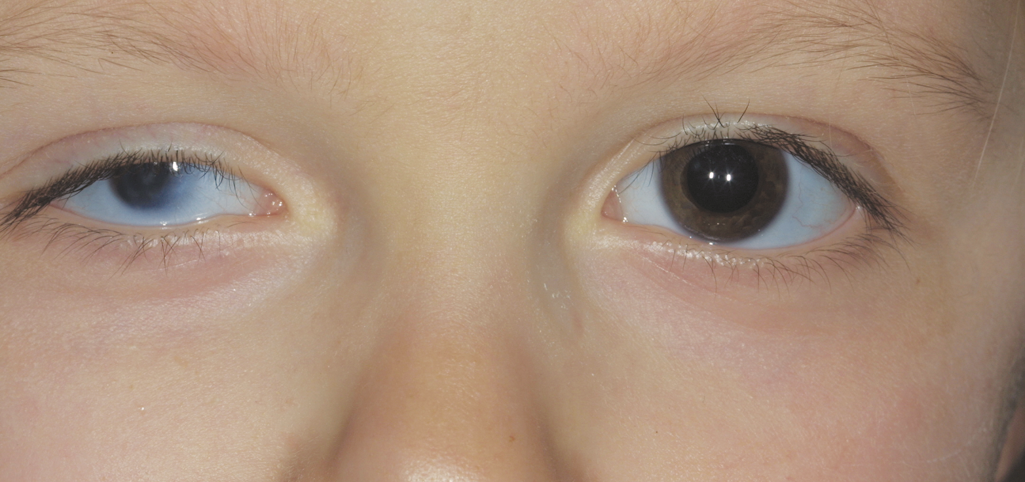
Figure 9. Microphthalmia, right eye. (© 2015 American Academy of Ophthalmology, www.aao.org.)
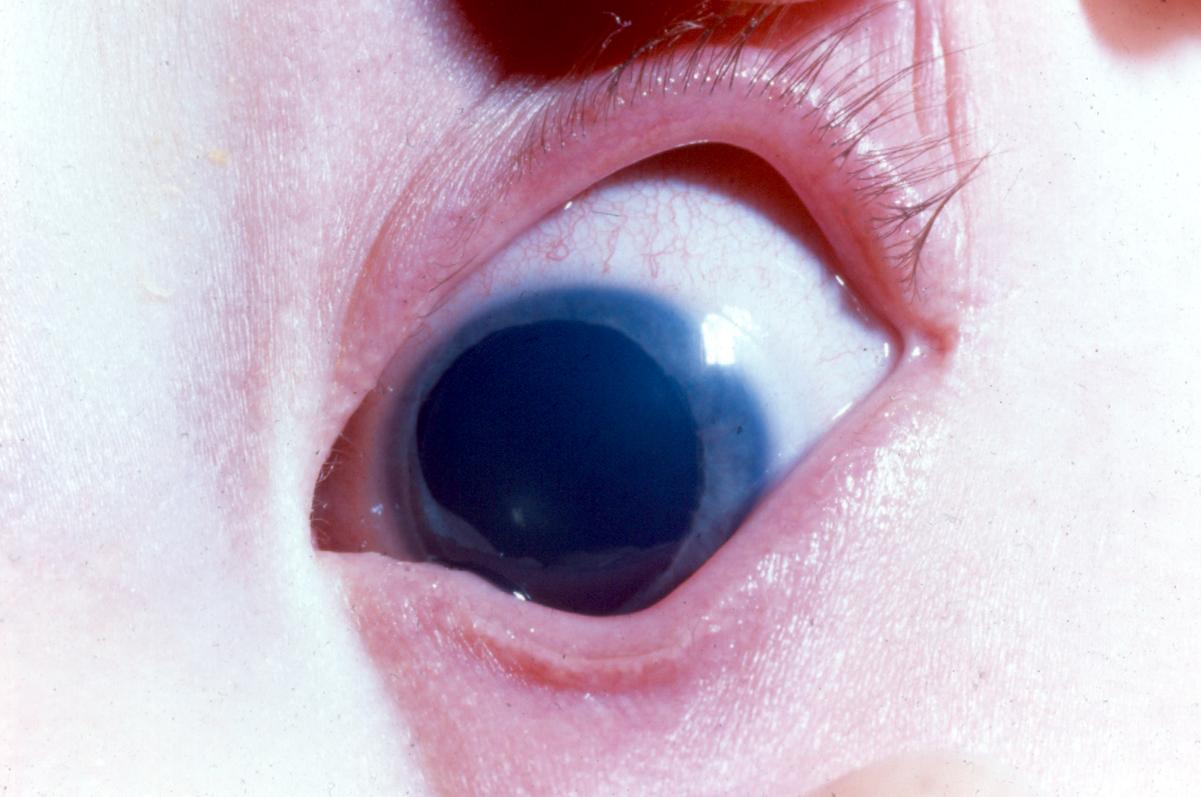
Figure 10. Congenital incomplete aniridia. (© 2015 American Academy of Ophthalmology, www.aao.org.
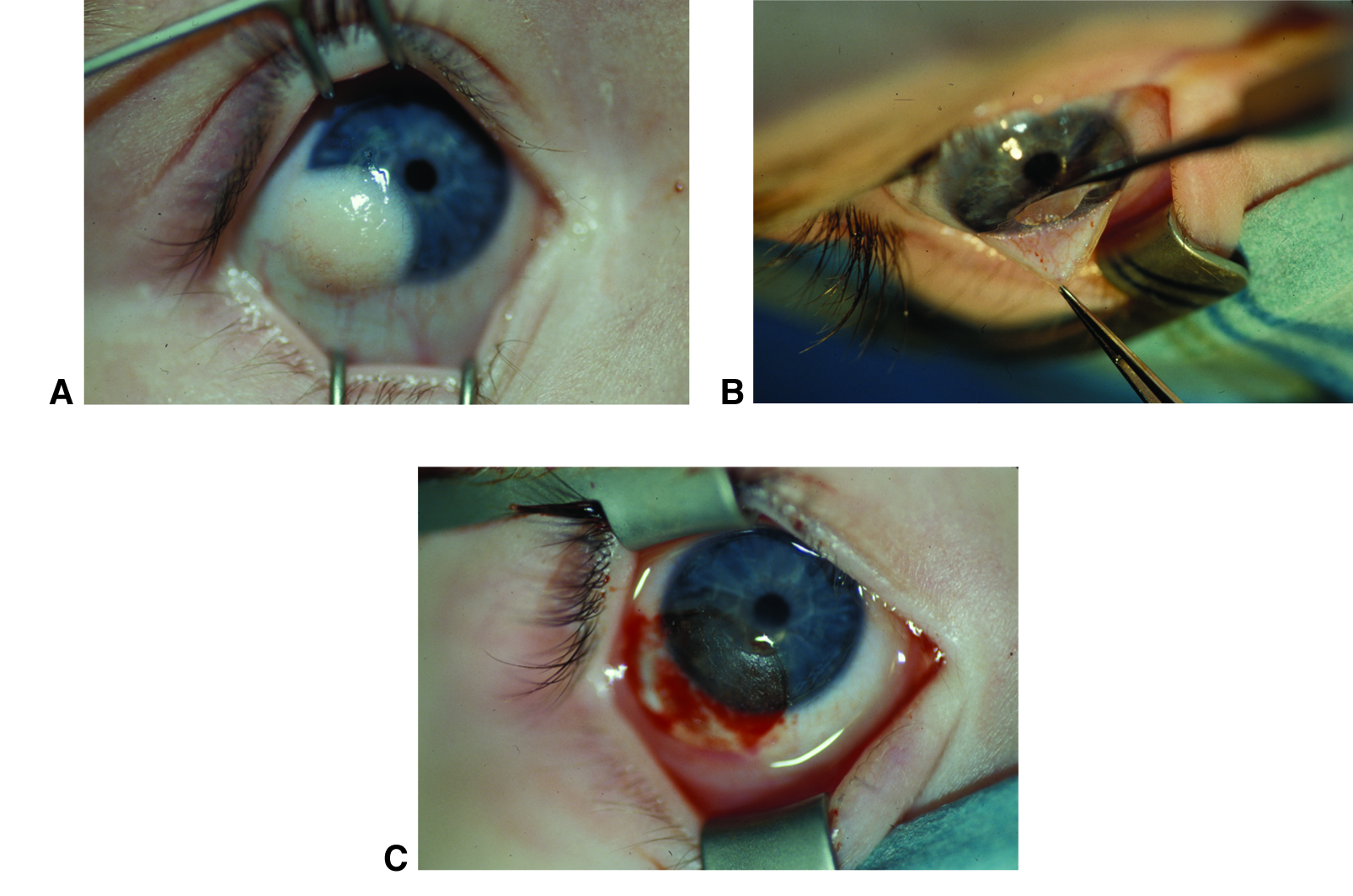
Figure 11. Corneal dermoid removal. Limbal dermoid before (A), during (B), and after (C) surgical excision. Although the cornea will likely heal with a scar, the scar will be less irritating and noticeable than the original dermoid. (© 2015 American Academy of Ophthalmology, www.aao.org. Courtesy of David A. Plager, MD.)
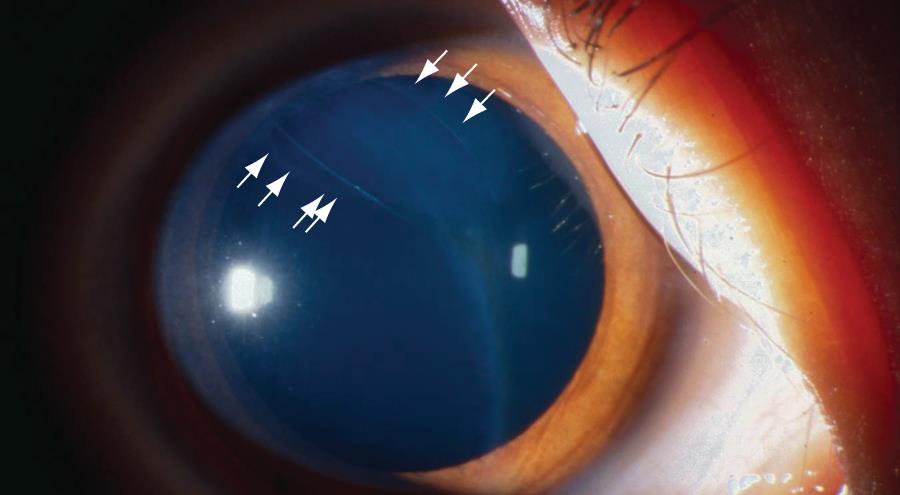
Figure 12. Breaks in Descemet membrane after forceps injury. (© 2015 American Academy of Ophthalmology, www.aao.org.)
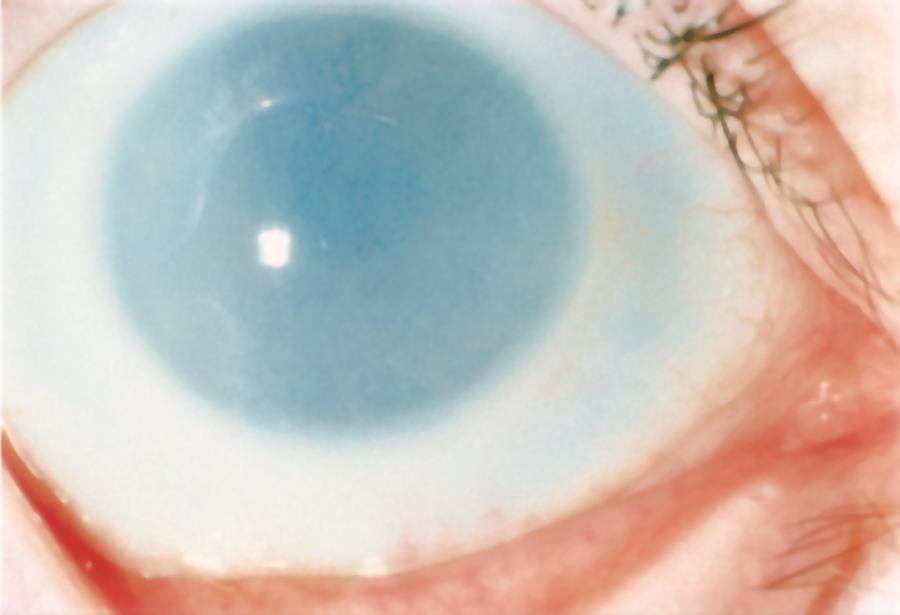
Figure 13. Diffuse corneal clouding in mucopolysaccharidoses type I. (© 2015 American Academy of Ophthalmology, www.aao.org.)
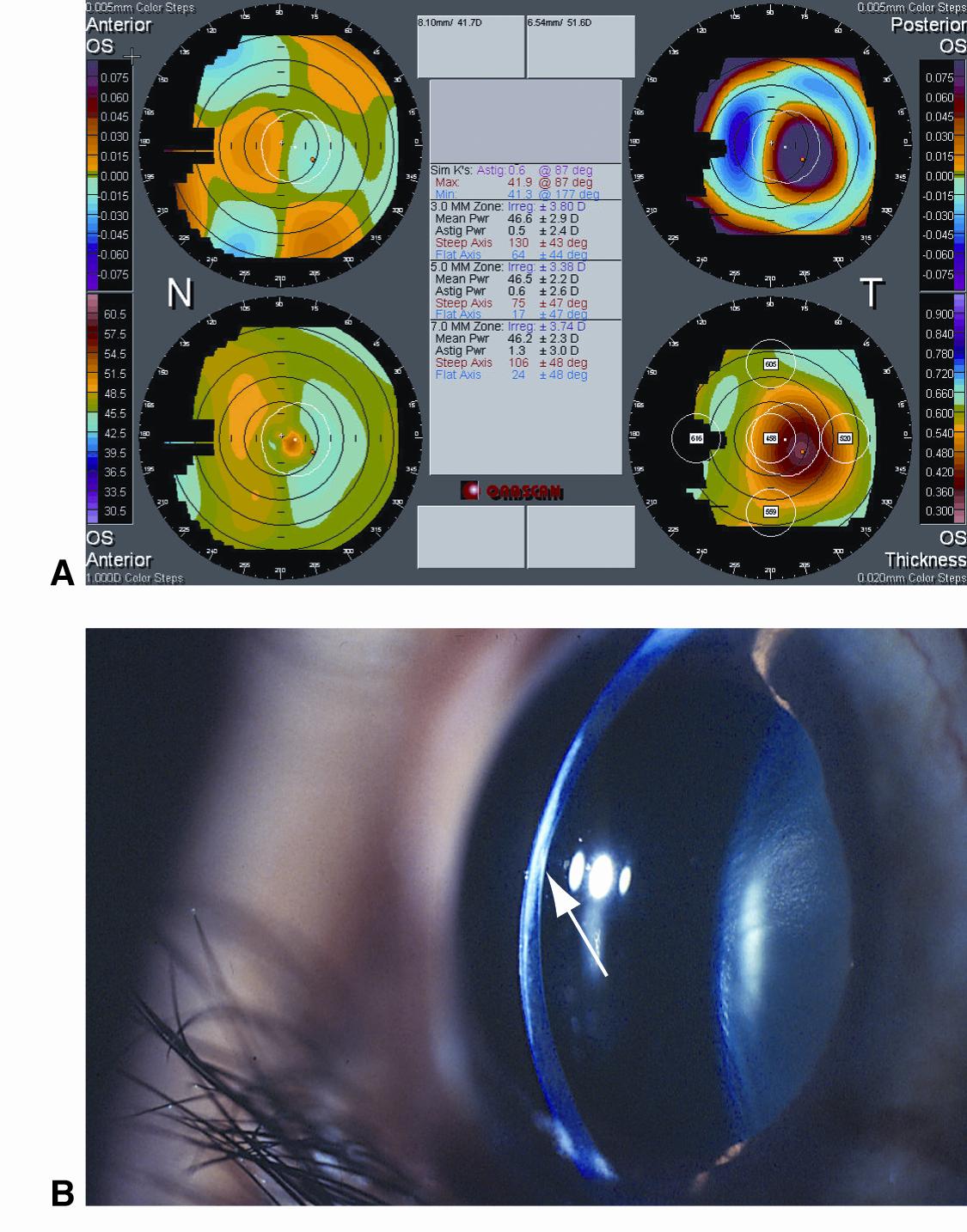
Figure 14. Posterior keratoconus. A. Scanning-slit corneal topography shows a nasally displaced anterior corneal apex (top left), temporal paracentral posterior corneal vaulting (top right), normal anterior keratometry reading (bottom left), and significant loss of stromal thickness (bottom right). B. A slit-lamp photograph shows loss of stromal thickness, stromal haze, and posterior corneal crater. (© 2015 American Academy of Ophthalmology, www.aao.org.)
REFERENCES
Bermejo E, Martínez-Frías ML. Congenital eye malformations: clinical-epidemiological analysis of 1,124,654 consecutive births in Spain. Am J Med Genet. 1998;75(5):497–504.
Bondunde OT, Ajibode, HA. Congenital eye diseases at Olabisi Onabanjo University Teaching Hospital, Sagamu, Nigeria. Niger J Med. 2006;15(3):291–4.
Burillon C, Durand L. Solid dermoids of the limbus and the cornea. Ophthalmologica. 1997;211(6):367–372.
Chang KC, Kwon JW, Han YK, Wee WR, Lee JH. The epidemiology of cosmetic treatments for corneal opacities in a Korean population. Korean J Ophthalmol. 2010;24(3):148–54.
Ciralsky J, Colby K. Congenital corneal opacities: a review with a focus on genetics. Semin Ophthalmol. 2007;22(4):241–246.
Comer RM, Daya SM, O’Keefe M. Penetrating keratoplasty in infants. J AAPOS. 2001;5(5):285–90.
Cotran PR, Bajart AM. Congenital corneal opacities. Int Ophthalmol Clin. 1992;32(1):93–105.
Dada T, Sharma N, Vajpayee RB. Indications for pediatric keratoplasty in India. Cornea. 1999;18(3):296–8.
Hovlykke M, Hjortdal J, Ehlers N, Nielsen K. Clinical results of 40 years of paediatric keratoplasty in a single university eye clinic. Acta Ophthalmol. 2014;92(4):370–7.
Kirkness CM, McCartney A, Rice NS, Garner A, Steele AD. Congenital hereditary corneal oedema of Maumenee: its clinical features, management, and pathology. Br J Ophthalmol. 1987;71(2):130–44.
Michaeli A, Markovich A, Rootman DS. Corneal transplants for the treatment of congenital corneal opacities. J Pediatr Ophthalmol Strabismus. 2005; 42(1):34–44.
Nischal KK. Congenital corneal opacities - a surgical approach to nomenclature and classification. Eye (Lond). 2007;21(10):1326–37.
Patel HY, Ormonde S, Brookes NH, Moffatt LS, McGhee CN. The indications and outcome of paediatric corneal transplantation in New Zealand: 1991–2003. Br J Ophthalmol. 2005;89(4):404–8.
Pediatric Ophthalmology and Strabismus. Basic and Clinical Science Course, Section 6, 2011–2012. San Francisco: American Academy of Ophthalmology.
Rezende RA, Uchoa UB, Uchoa R, Rapuano CJ, Laibson PR, Cohen EJ. Congenital corneal opacities in a cornea referral practice. Cornea. 2004;23(6):565–70.
Ricci B, Coppola A, Capobianco A, Ziccardi L. Penetrating keratoplasty in a newborn: case report and analysis of current surgical trends in Italy. Eur J Ophthalmol. 2008;18(2):290–3.
Shi W, Jin H, Li S, Liu M, Xie L. Indications of paediatric keratoplasty in north China. Clin Experiment Ophthalmol. 2007;35(8):724–7.
Yang LL, Lambert SR, Drews-Botsch C, Stulting RD. Long-term visual outcome of penetrating keratoplasty in infants and children with Peters anomaly. J AAPOS. 2009;13(2):175–80.