EPIDEMIOLOGY
Global Information
- Polypoidal choroidal vasculopathy (PCV) primarily affects pigmented individuals, especially Asians and African-Americans; however, it is now also recognized in the white population.
- Typically presents in 6th to 7th decade of life, though it may present earlier than other forms of macular degeneration
- Whites are affected to a lesser extent and with different clinical features
- Table 1 highlights the observed differences in PCV characteristics across ethnicities.
Table 1: Polypoidal Choroidal Vasculopathy Demographics and Characteristics |
|
Ethnicity
|
Mean Age
|
% Male
|
% Bilateral
|
% Central Macula Involving
|
% Peripapillary Involving
|
|
White
|
60-70
|
<50
|
20-80
|
25-68
|
35-75
|
|
Asian
|
60-70
|
>60
|
<10
|
>85
|
<15
|
Regional Information (North America)
- See Global Information above
- African Americans
- >90% of those with AMD may have PCV
DIFFERENTIAL DIAGNOSIS
RISK FACTORS/BIOMARKER
- Shared risk factors with AMD-CNV
- Age, male gender, smoking, hypertension, coronary artery disease, and hyperlipidemia
- Female gender was found to be a protective factor
- Large study in Korea: Age, body mass index (BMI), and higher education to be more strongly associated with PCV than AMD-CNV
- Ocular risk factors
- A history of CSC
- Systemic biomarkers
- C-reactive protein (CRP)
- Homocysteine
- Matrix metalloproteinase (MMP)
- Aqueous/vitreous biomarkers
- a. Interleukin-1b (IL-1b)
- VEGF
- Pigment epithelium derived factor (PEDF)
PATHOPHYSIOLOGY/DEFINITION
PCV is a neovascular and hemorrhagic disorder of the choroid.
Histology
- Degeneration of the walls of arterioles, capillaries, and large, dilated venules
- Large vascular channels and “polypoid” formations (Figure 9)
- Thickened capillary basement membranes
- Hyalinization of choroidal vessels
- Exudation of fibrin and plasma
Genetic Factors
- Gene HTRA1 is associated with PCV as well as neovascular AMD
- Complement factor H (CFH) variants rs3753394 and rs 800292 are associated with PCV
- CRP levels are higher in PCV patients and in AMD patients than in control patients
- I62V variant of CFH is associated with PCV in Japanese patients
- ARMS2 gene for mitochondrial photoreceptor protein is associated with PCV in Japanese patients
- CETP genetic variants to be associated with a high risk of PCV and is concordant with reports of elevated HDL as a risk factor for AMD, suggesting possible involvement of the high-density lipoprotein pathway
- Recent meta-analysis (2015)
- Complement cascade (CFH Y402H SNP rs1061170, CFH I62V SNP rs800292, C2 SNP rs547154, CFB SNP rs4151657, RDBP SNP rs3880457, and SKIV2L SNPs rs2075702 and rs429608)
- Inflammatory pathway (TNFRSF10A-LOC389641SNP rs13278062 and BEST- C4orf14-POLR2B-IGFBP7 SNP rs1713985)
- Extracellular matrix/basement membrane regulation pathway (ARMS2 A69S SNP rs10490924 and HTRA1 promoter SNP rs11200638) and lipid metabolism pathway (CETP SNP rs3764261)
- Previously associated with PCV (ARMS2, HTRA1, C2, CFB, ELN, LIPC, LPL, ABCA1, VEGF-A, TLR3, LOXL1, SERPING1, and PEDF) were not significantly associated with PCV in this meta-analysis
- Genotype associated with retreatment response
- ARMS2 LOC387715 rs10490924 variant: larger lesion size, higher likelihood of vitreous hemorrhage, and worse visual outcome after PDT or combination therapy
- ARMS2 A69S risk genotype :higher risk for second eye involvement
- CFH I62V polymorphism: associated with choroidal thickness
- ARMS2 A69S (rs10490924), HTRA1-rs11200638, PEDF gene polymorphism (SERPINF1 rs12603825): poor outcome after PDT
Vascular endothelial growth factor (VEGF) plays an uncertain role in the pathophysiology of PCV.
- VEGF has been localized to the retinal pigment epithelium (RPE) in patients with PCV
- Aqueous levels of VEGF were higher in PCV eyes compared to controls, but were significantly lower than aqueous VEGF levels in CNV-AMD
- VEGF has not been seen in the vascular endothelium on immunostaining of surgical specimens
- Anti-VEGF treatment has been well-reported to be effective in treating fluid associated with PCV; however, it has shown inconsistent results in resolving the polypoid vascular lesions
- While VEGF related angiogenesis processes were important in PCV pathogenesis, different angiogenesis pathways and cascades may be implicated as well
Natural Course
- Depends on factors including location (peripapillary vs macular), size of the lesion, and associated bleeding and exudation
- Polypoidal structures may also involute spontaneously
- Recurrent serosanguinous detachments of the RPE or neurosensory retina
- Microtears of RPE
- RPE atrophy
- Favorable course was seen in half of all patients with PCV while the other half had recurrences and eventual visual loss.
- 11% cumulative incidence of second-eye involvement in PCV in five years
Definition
- The Japanese Study Group of PCV
- Definite PCV: protruded orange-red elevated lesions on fundus examination and/or characteristic polypoidal lesions on ICGA
- Probable PCV: only an abnormal vascular network or occurrence of recurrent hemorrhagic and/or serous detachment of RPE were observed
- EVEREST study
- Subretinal focal ICGA hyperfluorescence with presence of at least one of the following
- Branching vascular networks (BVNs)
- Pulsatile polyp
- Nodular appearance on stereoscopic viewing
- Hypofluorescent halo
- Orange subretinal nodule on fundus photo
- Presence of massive submacular hemorrhage
SIGNS/SYMPTOMS
Clinical Characteristics
- Serosanguinous detachments of the RPE and neurosensory retina
- PCV lesions and choroidal neovascularization (CNV) (→The hallmark of PCV is the polypoidal lesion, which may be visible as an orange-red nodule on fundus examination)
- Typically peripapillary or central macula location
- Relatively preserved retinal architecture
- Better-than-expected visual acuity
- Pigment epithelial detachment associated with polypoid lesions
- Chronic lipid deposition
- Occasional spontaneous resolution with residual pigment mottling
- 2 types of PCV
- Exudative, characterized by serous PED and retinal detachment
- Hemorrhage, characterized by hemorrhagic PED and subretinal hemorrhage at the macula
Clinical characteristics may differ in white patients;
- Lesions are primarily peripapillary
- Drusen may also be seen in white patients
Imaging
Fluorescein angiogram (FA) (Figure 10)
- Branching vascular networks are often located in Bruch’s membrane (occult CNV)
- Polypoidal lesions can at times be difficult to see on FA. Lesions may manifest as either classic or occult leakage patterns
- Atrophy of RPE
Indocyanine green angiography (ICG) (Figure 11)
- Gold standard for diagnosis of PCV
- Choroidal vessel hyperpermeability
- Pulsatile polypoidal vessels
- Choroidal vascular networks
Optical coherence tomography (OCT) (Figure 12)
- Sub-RPE polypoidal lesions
- Pigment epithelial detachments
- Notch in the margin of large PED indicates the site of polypoidal lesions
- “Double layer sign”: two hyper-reflective lines represent separation of the RPE from Bruch’s membrane
- Higher rates of choroidal thickening
Fundus autofluorescence (FAF)
- Central hypo-autofluorescence with a circumferential hyper- autofluorescent ring corresponding to polypoidal lesions
- Granular hyperautofluorescence, corresponding to the branching choroidal vascular network
- Useful, non-invasive adjunct to ICGA and OCT for diagnosis and monitoring of treatment
OCT angiography
- The combination of en face and cross-sectional OCT angiographic images provides anatomical information about polypoidal structures
- More detailed view of type 1 CNV or branching vascular network complicated with PCV than ICGA
- Detection rate of polypoidal structures is lower than ICGA
- Only 50% of polyps were detected by OCT angiography from recent study performed in Korea
MANAGEMENT
- Photocoagulation
- Effective for treating extrafoveal polyps, but success rate is variable
- Persistent exudation from branching vascular network reported in 28.5%-37.7%
- Yuzawa reported that only 10% of eyes worsened if both the polyp and network were lasered, compared to 54% of eyes worsened after laser to polyps only. However, lasering the whole network is often impossible due to the large area involved
- Combined with anti-VEGF, photocoagulation has been shown to confer additional benefit in reducing exudation and hemorrhage from the network, while focal laser can be targeted mainly to the polyps.
- Treatment of the entire lesion is effective in resolving exudative lesions and preventing further vision loss
- Treatment of only the polypoid lesion does not prevent recurrence or progression
- Laser photocoagulation is reserved for extrafoveal polyps. Treating the entire lesion including the branching vascular network may not always be possible.
- Anti-VEGF
- The pathophysiology of polypoidal lesions may not be a VEGF-driven process
- Intravitreal bevacizumab has been shown to be effective treatment for fluid associated with PCV, but has not been shown to improve polypoidal lesions (25%-40% of polypoidal lesion regression rate)
- BVNs do not appeared to be affected by anti-VEGF monotherapy
- Early results of intravitreal aflibercept suggest that aflibercept may be more effective at achieving polypoidal lesion closure (55%-70%) than other ant-VEGF agents.
- Photodynamic Therapy (PDT)
- PDT has been shown to be effective in preserving or improving visual acuity (80% of patients)
- PDT may be more effective for PCV than for wet AMD
- PDT has been shown to be effective in closure of polypoidal lesions (>70%), but, ineffective in causing regression of the BVN
- Retreatment rates are much lower than anti-VEGF therapy
- Complications: acute vision loss, progressive RPE atrophy, RPE tears, subretinal hemorrhage and vitreous hemorrhage
- Combination therapy (PDT+anti-VEGF)
- Anti-VEGF may also counteract the potential upregulation of VEGF after the application of PDT
- EVEREST study, the combination arm achieved the best angiographic and visual outcome, with the highest proportion of complete polypoidal lesion regression (78%) and highest mean gain in vision (+10.9 letters)
- Fujisan study reported the similar visual and anatomical improvements at 1 year between initial and deferred PDT combined with ranibizumab
- Anti-VEGF vs PDT:
- Many studies reporting up to 12-month follow-up have described stabilization of vision and improvement of exudation
- Inoue et al reported better vision at month 18 and 24 in eyes treated with intravitreal ranibizumab compared to those treated with PDT
- LAPTOP study based in Japan (Phase IV, prospective RCT) reported better visual outcomes in eyes treated with ranibizumab than eyes treated with PDT at month 12 and month 24
- Concern remains related to incomplete polyp regression, which has been thought to lead to recurrence
- Higher recurrence rate has been reported in anti-VEGF-treated eyes (64.9%) compared to PDT treated eyes (47.4%) at 2 years in a study based in Korea
- EVEREST study: A multicenter RCT reported higher polyp closure rate in eyes treated with PDT (77.8% combined with ranibizumab and 71.4% PDT alone) compared to eyes treated with ranibizumab alone (28.6%). However, visual acuity gain in the ranibizumab group was numerically higher than PDT monotherapy group (9.2 letters vs 7.5 letters, not statistically significant)
- Some centers (particularly in Japan) recommend reserving PDT to eyes with poor vision to minimize the risk of visual deterioration that may be seen in PDT
- Other treatment options for PCV
- Radiation therapy: stereotactic radiation therapy (SRT) has been shown 83% polypoidal lesion regression rate and improvement of 7.6 ETDRS letters at month 12 after a single dose of 16-Gy SRT and PRN ranibizumab in one study
- Management of submacular hemorrhage
- 20%-60% of cases, PCV has been found to be the cause of submacular hemorrhage
- Pneumatic displacement and recombinant tissue plasminogen activator (rtPA) injection with vitrectomy
- Anti-VEGF monotherapy alone may be effective for thin submacular hemorrhage
- Peripapillary PCV
- Anti-VEGF monotherapy represent the safest treatment option
- The optimal therapy for PCV is still unclear. Currently there are two clinical trials comparing anti-VEGF alone with combination therapy (anti-VEGF and PDT).
- EVEREST 2: ranibizumab monotherapy vs. ranibizumab+PDT
- PLANET: aflibercept monotherapy vs. aflibercept with adjunctive photodynamic therapy
Treatment Algorithm
- Workup: clinical history, dilated fundus exam (FA/ICG)
- If visual acuity is 20/30 or better: Observation, though treatment initially with anti-VEGF may be considered in patients with good visual acuity but significant exudate
- If visual acuity is 20/40 or worse: Consider treatment for symptomatic patients
- PDT is often effective initial treatment for PCV where available
- If the polypoidal lesion is associated with significant exudate or fluid, consider PDT + anti-VEGF therapy
- Recurrent associated fluid that is visually significant and responsive to anti-VEGF intravitreal injection may require repeat anti-VEGF injections to maintain stable vision
- If lesions are not responsive to repeat injections of an initial anti-VEGF agent, a switch to a different agent may be effective (eg, switching from ranibizumab to aflibercept)
- If PDT is not an available treatment option, laser photocoagulation of polypoidal lesions outside of the foveal avascular zone may be an effective treatment strategy; however, laser photocoagulation has been associated with macular hemorrhage and should be performed only if the entire lesion can be treated.
IMAGE LIBRARY
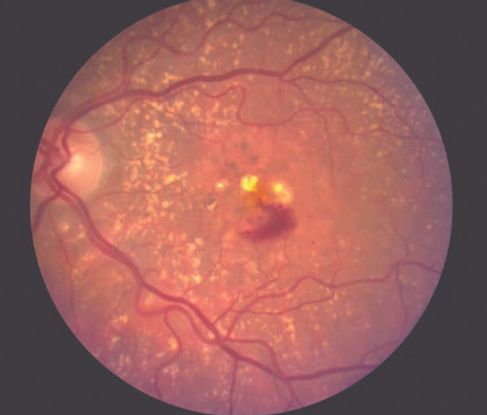
Figure 1. Age-related macular degeneration (AMD) ©American Academy of Ophthalmology, 2014.
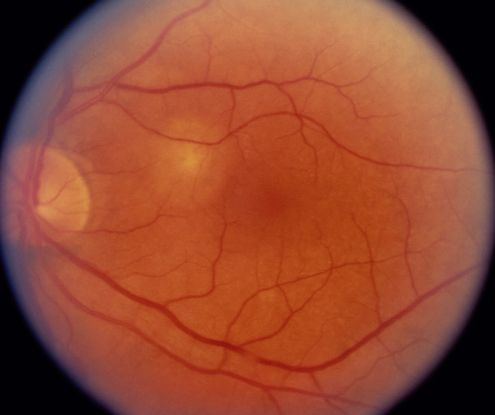
Figure 2. Choroidal neovascularization (CNV) ©American Academy of Ophthalmology, 2014.
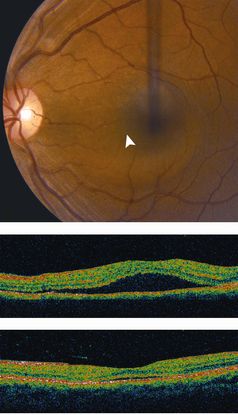
Figure 3. Central serous chorioretinopathy (CSC) ©American Academy of Ophthalmology, 2014.
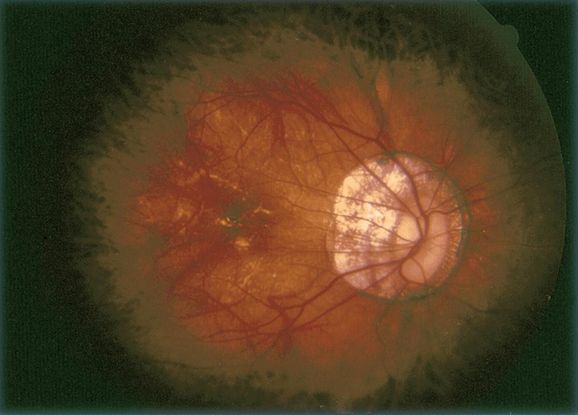
Figure 4. Pathologic myopia ©American Academy of Ophthalmology, 2014.
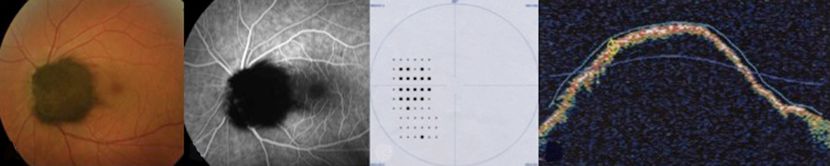
Figure 5. Melanocytoma ©American Academy of Ophthalmology, 2014.
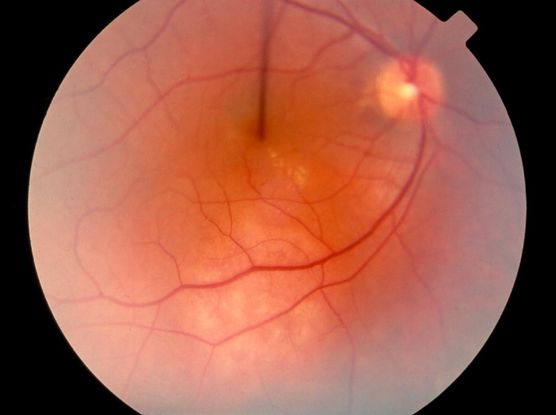
Figure 6. Choroidal tumors (hemangioma, melanoma, etc) ©American Academy of Ophthalmology, 2014.
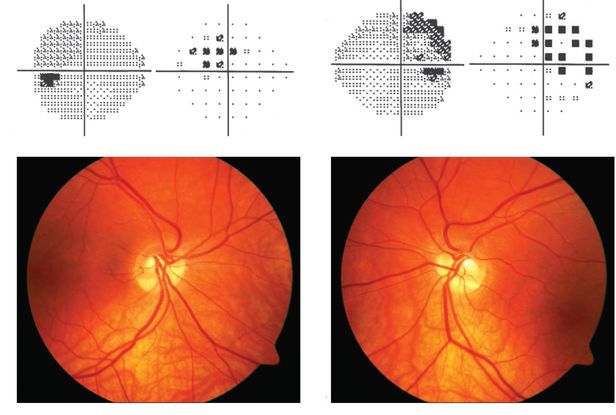
Figure 7. Tilted disc syndrome ©American Academy of Ophthalmology, 2014.
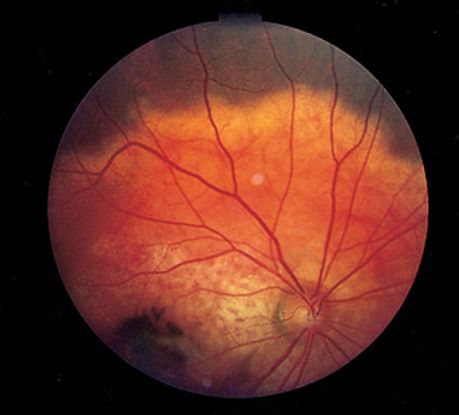
Figure 8. Choroidal osteoma ©American Academy of Ophthalmology, 2014.
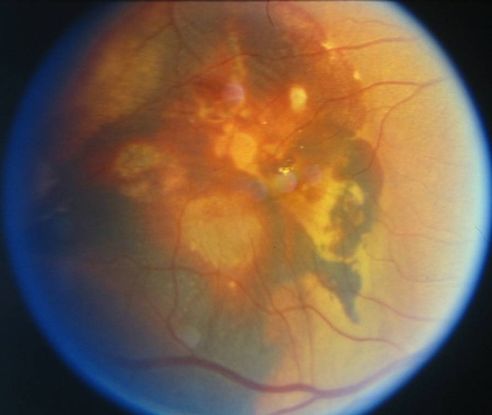
Figure 9. Large vascular channels and “polypoid” formations ©American Academy of Ophthalmology, 2014.
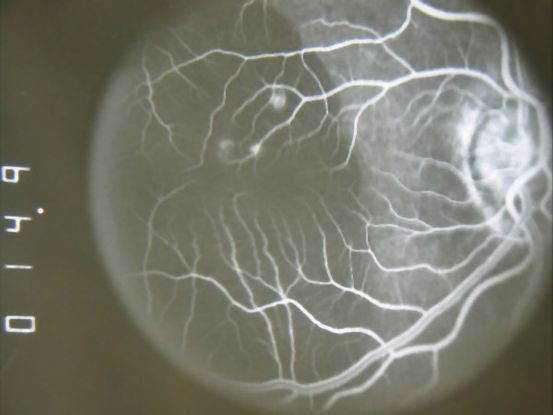
Figure 10. Fluorescein angiogram (FA) ©American Academy of Ophthalmology, 2014.
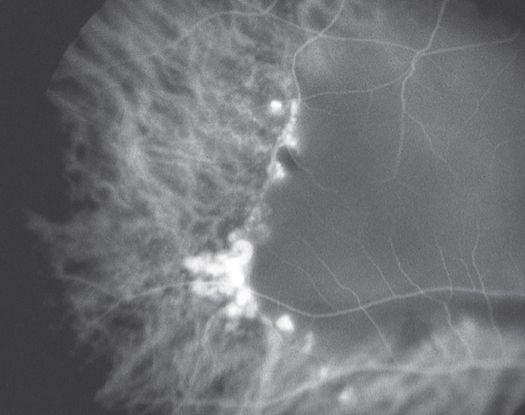
Figure 11. Indocyanine green angiography (ICG) ©American Academy of Ophthalmology, 2014.
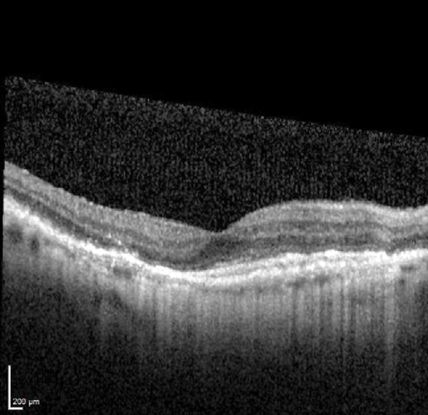
Figure 12. Optical coherence tomography (OCT) ©American Academy of Ophthalmology, 2014.
REFERENCES
Ahuja RM, Stanga PE, Vingerling JR, Reck AC, Bird AC. Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelium detachments. Br J Ophthalmol. 2000; 84(5): 479-484.
Bozzoni Pantaleoni F, Magliari Galante V, Da Dalt S, Pecorella I. Localizing polypoidal choroidal vasculopathy for laser treatment. Acta Ophthalmol Scand. 2007; 85(4): 456-458.
Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009; 148 (1): 70-78.
Gemmy Cheung CM, Yeo I, Li X, et al. Argon laser with and without anti- vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol . 2013; 155: 295- 304 e291.
Gomi F, Sawa M, Sakaguchi H, et al. Efficacy of intravitreal bevacizumab for choroidal vasculopathy. Br J Ophthalmol. 2008; 92(1): 70-73.
ImamuraY, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal Choroidal Vasculopathy: A Review. Surv Ophthalmol. 2010: 55(6):501-515.
Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term efficacy of intravitreal aflibercept in treatment naïve patients with polypoidal choroidal vasculopathy. Retina. 2014; 34 (11):2178-2184.
Jeon S, Lee WK, Kim KS. Adjusted retreatment of polypoidal choroidal vasculopathy after combination therapy: results at 3 years. Retina. 2013; 33: 1193-1200.
Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol. 2007; 144(4): 608- 612.
Kwok AK, Lai TY, Chan CW, Neoh EL, Lam DS. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002; 86(6): 892-897.
Lafaut BA, Leys AM, Snyers R, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000; 238(9): 751-759.
Lee KY, Vithana EN, Mathur R, et al. Association analysis of CFH, C2, BF, and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(6): 2613-2619.
Lee MW, Yeo I, Wong D, Ang CL. Argon laser photocoagulation for the treatment of polypoidal choroidal vasculopathy. Eye (Lond.). 2009; 23(1):145-148.
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007; 144(1): 15- 22.
Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinico-pathological findings of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(11): 4729-4737.
Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002; 86(10): 1093-1098.
Reche-Frutos J, Calvo-Gonzales C, Donate-Lopez J, Garcia-Feijoo J, Leila M, Garcia-Sanchez J. Short- term anatomic effect of ranibizumab for polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2008; 18(4): 645- 648.
Saito M, Kano M, Itagaki K, Oguchi Y, Sekiryu T. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014; 34(11):2192-2201,
Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121: 1392- 1396.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15: 100-110.
Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002; 86(3): 321-327.
Tsujikawa A, Sasahara M, Otani A, et al. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007; 143(1): 102-111.
Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003; 47(4): 379-384.
CONTRIBUTORS
Executive Editor:
R. V. Paul Chan, MD, FACS, Weill Cornell Medical College, New York, New York
Associate Editors:
Jeff Pettey, MD, University of Utah Department of Ophthalmology and Visual Sciences, John Moran Eye Center's Residency Program Director
Grace Sun, MD, Weill Cornell Eye - Lower Manhattan, Weill Cornell Medical College Residency Program Director
Assistant Editors:
Samir Patel, BS, Weill Cornell Medical College, New York, New York
Peter Coombs, MD, Weill Cornell Medical College; New York, New York
American Academy of Ophthalmology
P.O. Box 7424
San Francisco, CA 94120-7424
415.561.8500
Copyright © 2016 American Academy of Ophthalmology®. All Rights Reserved.