A preclinical study conducted at the University of Birmingham has found that topically applied cell-penetrating peptide (CPP) molecules can deliver anti-VEGF agents to the retina. The findings, published in Investigative Ophthalmology & Visual Science, could portend a paradigm shift in the treatment of AMD, replacing the standard of burdensome intravitreal injections with eye drops.
Cell-penetrating peptides are short-sequence peptides used in previous applications to chaperone small molecules into retinal cells following subretinal injection. However, their capacity for delivering large drug molecules, such as anti-VEGFs, through the many tissue barriers between the eye’s surface and posterior segment was unknown.
The team tested topical administration of ranibizumab and bevacizumab complexed with CPPs on rat eyes in vivo and on pig eyes ex vivo. They found clinically relevant levels of each anti-VEGF present in rat retinal tissue within 30 minutes, with titers increasing for 1 hour. Similar bioavailability was detected in the porcine vitreous and retina after 45 minutes, suggesting that CPPs are effective for carrying drugs to the posterior segment of a human-sized eye. Because the drug was mostly cleared from retinal tissues after 24 hours, the authors hypothesized that a daily dosing regimen would be required to provide a therapeutic effect.
Subsequently, the authors compared the efficacy of twice daily topical anti-VEGF drops with a single intravitreal injection or systemic dexamethasone in a mouse model of choroidal neovascularization (CNV).
After 10 days, mice treated with CPPs showed significant reductions in the size of laser-induced CNV lesions compared with untreated eyes and those that received uncomplexed anti-VEGFs. Furthermore, these anatomical improvements were similar to those in the steroid and intravitreal groups, suggesting that the drops could be as effective as traditional anti-VEGF injections. The study also found no evidence of CPP toxicity to ocular cells. Cultures of mixed rat retinal cells, human retinal pigment epithelium cells and human corneal fibroblasts showed no difference in survival when exposed continuously to various dosages of CPP for 3 days.
If the mechanism translates to human eyes, lead researcher Felicity de Cogan, PhD, believes the treatment could empower AMD patients while greatly reducing the cost of treatment. “The CPP-drug has the potential to have a significant impact on the treatment of AMD by revolutionizing drug-delivery options. [It] also has potential application to other chronic ocular diseases that require drug delivery to the posterior chamber of the eye,” she said.
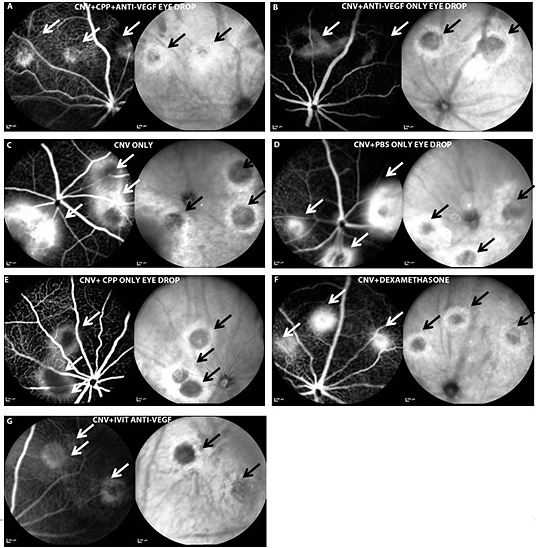
In vivo scanning laser ophthalmoscope (SLO) images of fluorescein angiography (FA) (left) and infrared imaging (IR) (right) of mouse retinae after laser induction of CNV wounds. In both FA (black arrows) and IR (white arrows) images, CNV laser lesions are apparent as halos of leaked fluorescein from retinal blood vessels, with light areas of hyperfluorescence surrounding a central dark hypofluorescent core (scale bar: 200 μm). Image and text from the Association for Research inVision and Ophthalmology.