Since a 2003 survey on practice styles and preferences of American Society of Cataract and Refractive Surgery (ASCRS) members found that hydrophobic acrylic had been the preferred optic material since 1998,1 the U.S. market has seen increased variability in the materials used to manufacture foldable intraocular lenses (IOLs). The foldable IOLs available in the U.S. are manufactured from hydrophobic acrylic, silicone and hydrophilic acrylic materials, with an increasing number of hydrophilic acrylic designs now available. These include Centerflex (Rayner), Softec HD (Lenstec) and Akreos (Bausch & Lomb). This article discusses the pros and cons to consider when selecting or recommending an IOL.
Modern foldable IOLs generally are manufactured and marketed as having square posterior optic edges for PCO prevention. However, all square edges in the market are not the same. For example, glistenings have a stronger association with hydrophobic acrylic materials, which exhibit different tendencies to glistening formation than other materials.
Calcification is associated with hydrophilic acrylic materials, although the posterior optic surface of silicone lenses may exhibit calcification in eyes with asteroid hyalosis. However, I know of no confirmed cases of calcification of hydrophobic acrylic lenses described in the literature, which suggests that postoperative calcification is not an issue with hydrophobic acrylic materials.
Studies generally have shown postoperative inflammatory reactions to be low grade and clinically insignificant with modern foldable IOLs, although the intensity and duration of responses may vary with the IOL material.
Multitude of materials
Biomaterials (polymers) used for the manufacture of IOL optics can be divided into two major groups: acrylic and silicone.2 Acrylic lenses can be further divided into: rigid, such as those manufactured from poly(methyl methacrylate) (PMMA); and foldable, manufactured from hydrophobic acrylic materials, including AcrySof (Alcon Laboratories) and Sensar (Advanced Medical Optics), or manufactured from hydrophilic acrylics, also known as hydrogels, including Centerflex and Akreos.
Each foldable acrylic lens design is manufactured from a different copolymer acrylic with a different refractive index, glass transition temperature (above this temperature the polymer exhibits flexible properties and below it remains rigid), water content, mechanical properties and other attributes.
Hydrophobic acrylic lenses, as well as silicone lenses, have very low water content, generally less than 1 percent. However, more recently, hydrophobic acrylic materials with higher water content (approximately 4 percent) have become available. These include Bausch & Lomb's enVista lens.
Most of the currently available hydrophilic acrylic lenses are manufactured from copolymers with water content from 18 to 38 percent.
The first silicone material used in the manufacture of IOLs was poly(dimethyl siloxane), which has a refractive index of 1.41. The latest generations of silicone materials have higher refractive indexes.
However, the refractive index is higher in foldable acrylics than silicones, at 1.47 or greater, so that acrylic lenses are thinner than silicone lenses with the same refractive power. While foldable acrylics display glass transition temperatures of around room temperature, the glass transition temperature of silicones can be significantly below room temperature.
Uveal biocompatibility
The uvea's reaction to the IOL is its biocompatibility. The blood-aqueous barrier is disrupted during cataract surgery with IOL implantation, with proteins and cells released in the aqueous humor. Proteins then adsorb on the IOL surface, and this will influence subsequent cellular reactions on the IOL.2
Clinical studies have compared the inflammatory reaction after implantation of IOLs manufactured from different biomaterials.3-6 Comparison of postoperative flare values showed no clinically relevant differences among foldable biomaterials in patients with or without associated history of uveitis. Although absolute flare values and cell counts in eyes with uveitis were found to be higher than in control eyes in one study, primarily because of damaged blood-aqueous barriers, postoperative recovery was similar.
Monocytes and macrophages migrate through uveal vessel walls into the aqueous humor and onto the IOL surface. Monocytes (lymphocytes) transform into small round cells and macrophages into epithelioid and foreign-body giant cells that are responsible for the phagocytosis of debris, bacteria and foreign material. These cells reflect a natural immunological process in a foreign-body reaction.
In a study by Abela-Formanek et al. evaluating uveal biocompatibility of hydrophilic acrylic, hydrophobic acrylic and silicone IOLs, the hydrophobic acrylic IOLs showed the highest incidence of late foreign-body cell reaction (AcrySof, 30 percent; AR40, 17 percent), followed by the hydrophilic acrylic (MemoryLens, 8 percent; Hydroview, 4 percent) and silicone (CeeOn 920, 4 percent; CeeOn 911A, 0 percent) (P = 0.0044).3 In the same study, the cellular reaction was found to be low grade and clinically insignificant in all cases. Giant cells usually degenerate and detach from the IOL surface, and only an acellular proteinaceous membrane surrounds the IOL, isolating it from the surrounding ocular tissues.
Capsular biocompatibility
The reaction of lens epithelial cells (LECs) and the capsule to IOL material and design is its capsular biocompatibility. The outcome of LEC outgrowth, anterior capsule opacification (ACO) and posterior capsule opacification (PCO) was evaluated in the same above-mentioned study.3 The highest incidence of LEC outgrowth on the anterior IOL surface (originating from the anterior capsular rim) was in the hydrophilic acrylic group (Hydroview, 85 percent; MemoryLens, 27 percent) followed by the hydrophobic acrylic group (AcrySof, 4 percent; AR40, 3 percent). No silicone IOL had LECs on the anterior surface. The difference among IOL groups was significant (P = 0.0001). ACO was more predominant with hydrophobic IOLs.
Other studies have shown more fibrosis of the anterior capsule and ACO in association with silicone lenses, especially the plate designs.7,8 Abela-Formanek's study,3 as well as others,9 have shown that PCO was lower with IOLs with a square edge on the posterior optic surface, regardless of IOL material.
IOL design features and PCO prevention
A square edge on the posterior optic surface appears to be the most important IOL design feature for PCO prevention. According to various studies, the preventive PCO effect associated with the square edge may be due to a mechanical barrier effect,10 the contact inhibition of migrating LECs at the capsular bend created by the edge,11 the higher pressures exerted by IOLs with a square-edged optic profile on the posterior capsule,12 or combinations of all three factors.
In a series of experimental studies that included 19 hydrophobic acrylic lenses, 11 silicone lenses and 47 hydrophilic acrylic lenses, the area above the posterior-lateral edge was measured in terms of area deviating from a perfect square.13,14 Demonstrating that not all square edges are the same, there was a large variation in the deviation area from a perfect square, not only between different IOL designs from the same material group but also between different powers of the same design (see Figure 1).
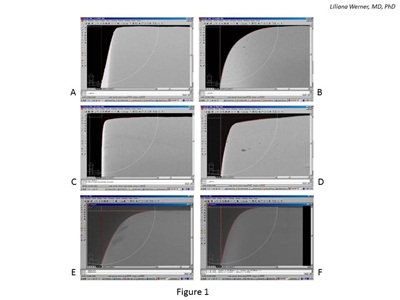
Figure 1: Scanning electron photomicrographs used in the evaluation of the area deviating from a perfect square of six +20.0 D IOLs. All lenses are marketed as having square optic edges. A and B: Hydrophobic acrylic lenses. C and D: Silicone lenses. E and F: Hydrophilic acrylic lenses.
An environmental scanning electron microscopic (SEM) technique was used to evaluate the hydrophilic acrylic lenses under low vacuum conditions, preventing dehydration.14 The microedge structure of modern hydrophilic IOLs, most of which have water content around 26 percent, may be significantly modified by the vacuum required in standard SEM procedures. The deviation area measurement values of hydrophilic acrylic lenses as a group were higher than those reported for hydrophobic acrylic or silicone lenses; therefore, the edges were overall rounder. The differences among the three groups of materials were statistically significant.
This type of evaluation is useful in elucidating clinical findings. Various clinical studies are aimed at comparing PCO rates between hydrophobic and hydrophilic IOLs marketed as having square optic edges.15,16 The results have been generally less favorable for hydrophilic acrylic lenses, and investigators have concluded that this was due to the characteristics of the IOL material. However, it is possible that the square edges of the lenses evaluated in those studies were not really comparable in terms of area deviating from a perfect square.
Glistenings
Glistenings are fluid-filled microvacuoles that form within the IOL optic when the lens is in an aqueous environment (see Figure 2). This finding was discussed in an extensive review paper recently published in the peer-reviewed literature.17
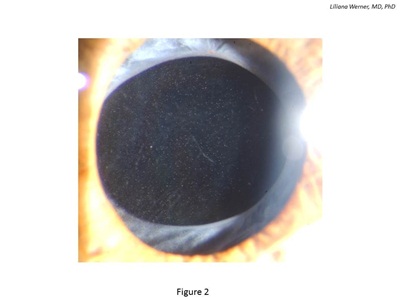
Figure 2: Clinical photograph of an eye implanted with a hydrophobic acrylic IOL exhibiting intraoptical glistenings.
Glistenings can be observed with any type of IOL, although most available literature describes them in association with hydrophobic acrylic lenses. However, experimental and clinical studies suggest that different hydrophobic acrylic lenses currently on the market exhibit different tendencies regarding glistenings.
Factors that may influence the formation of glistenings include IOL material composition, manufacturing technique, and packaging; associated conditions, such as glaucoma; conditions leading to breakdown of the blood-aqueous barrier; and concurrent use of ocular medications. While the exact impact of glistenings on postoperative visual function, as well as their evolution in the late postoperative period, remains a matter of controversy, IOL explantation due to glistenings rarely has been reported.
Advanced Vision Science (AVS), a subsidiary of Santen Pharmaceutical Co., Ltd. (Osaka, Japan), recently has developed a new hydrophobic acrylic material, which is a copolymer of hydroxyethyl methacrylate, polyethylene glycol phenyl ether acrylate and styrene, cross-linked with ethylene glycol dimethacrylate. The material has a refractive index of 1.54 and water content of approximately 4 percent. It was approved for use in the U.S. in February 2009 (and subsequently licensed to Bausch & Lomb) and in Japan in 2008.18
The AVS IOL is the only hydrophobic acrylic lens packaged in solution. The current version, packaged in 0.9% saline and gamma-sterilized, was not associated with any glistening in clinical studies.17
Calcification
Postoperative optic opacification of modern hydrophilic acrylic IOLs has been a significant complication leading to IOL explantation since 1999 19 (see Figure 3). Results of the 2001 survey sent to ASCRS members showed that hydrophilic acrylic IOLs were the most frequently explanted lenses (28 percent). In the majority of the cases (98 percent), explantation was required because of optic opacification.20
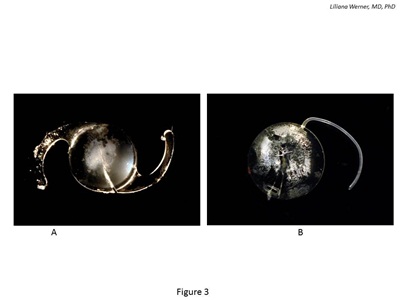
Figure 3: Gross photographs of IOLs explanted because of postoperative calcification. A: Hydrophilic acrylic lens exhibiting calcification after DSAEK. The calcified deposits are on the anterior optic surface/subsurface and are localized to the central part of the optic. B: Silicone lens exhibiting calcified deposits on the posterior optic surface. The lens was explanted from an eye with asteroid hyalosis.
Studies that have used histopathological, histochemical, electron microscopic, elemental or molecular surface analytical techniques have demonstrated that this opacification was related to calcium/phosphate precipitation on and/or within the lenses.21-26 The four major designs manufactured in the U.S. involved in the problem were the Hydroview (Bausch & Lomb), MemoryLens (Ciba Vision), SC60B-OUV (Medical Developmental Research) and Aqua-Sense (Ophthalmic Innovations International).
Although in many cases it was difficult to determine when optic opacification was first observed, the lenses were on average explanted during the second year after implantation. The opacification was not associated with anterior segment inflammatory reaction, and Nd:YAG laser was not effective at removing calcified deposits from the lenses.
Calcification of hydrophilic acrylic lenses appears to be a multifactorial problem. Factors related to IOL manufacture, packaging, surgical techniques and adjuvants, and patient metabolic conditions are among those that may be implicated.
As the exact combination of factors and sequence of events ultimately leading to lens calcification is still unknown, further research is warranted. This requires a multidisciplinary approach, which is further complicated by the fact that detailed manufacturing procedures are considered proprietary, and some IOL designs have different commercial names in different countries.
In the meantime, surgeons must be able to recognize this condition during clinical examination, since missing it can lead to potentially avoidable surgical procedures and increased risk of complications after repeated interventions.25 Explantation/exchange of the opacified/calcified IOL is to date the only treatment.
There also have been sporadic reports of postoperative IOL calcification involving other hydrophilic acrylic lenses, including designs manufactured in Europe.19,26 These situations mostly have been related to complicated cases, with significant postoperative inflammation, multiple surgical procedures, and intraocular use of adjuvants, such as tissue plasminogen activator (for severe fibrinous reaction), silicone oil, air or gas. The onset of signs and symptoms may occur much earlier than in the cases of dystrophic calcification mentioned above, and the pattern of calcium deposition may vary. In cases following surgical procedures involving the use of air or gas inside of the eye (e.g., Descemet stripping endothelial keratoplasty), the pattern of calcification appears to be more superficial, on or close to the anterior surface of the lens and confined to the pupillary area.
Calcification in asteroid hyalosis
Calcified deposits leading to significant opacification requiring explantation also have been observed on the surface of silicone IOLs in eyes with asteroid hyalosis.27-30 The composition of asteroid bodies was found to be similar to that of hydroxyapatite (calcium and phosphate). Four cases were initially reported in the literature, all with silicone plate lenses in patients with unilateral asteroid hyalosis.27,28 Whitish deposits appeared only on the posterior optic surface of the lens late postoperatively.
Two of the four reported patients had diabetes. In two of the cases, the deposits were noted before Nd:YAG laser capsulotomy was performed. Fast re-accumulation of the deposits on the posterior surface of the lenses was described after the procedure. In the other two cases, it is not clear whether or not the deposits were present before the Nd:YAG procedure.
Later, my colleagues and I described the first similar case related to a three-piece silicone lens in a patient with bilateral asteroid hyalosis.29 Only the silicone lens exhibited calcification; the hydrophobic acrylic lens implanted in the contralateral eye developed no opacities after six years. More recently, we reported on 16 new cases involving eight designs of silicone lenses manufactured from five different silicone materials.30
It is unclear why there are so few reported cases of opacification despite the numerous silicone IOLs that have likely been implanted in patients with asteroid hyalosis since these IOLs became available in the 1980s. In light of the increasing number of opacified silicone lenses in these eyes involving a variety of IOL designs, surgeons may consider adding our findings to the list of pros and cons they consider when selecting or recommending an IOL.
References
- Leaming DV. Practice styles and preferences of ASCRS members-2003 survey. J Cataract Refract Surg. 2004;30:892-900.
- Werner L. Biocompatibility of intraocular lens materials. Curr Opin Ophthalmol. 2008;19:41-49 (Review).
- Abela-Formanek C, Amon M, Schild G, Schauersberger J, Heinze G, Kruger A. Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. J Cataract Refract Surg. 2002;28:50-61.
- Abela-Formanek C, Amon M, Schauersberger J, Kruger A, Nepp J, Schild G. Results of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in uveitic eyes with cataract: comparison to a control group. J Cataract Refract Surg. 2002;28:1141-1152.
- Abela-Formanek C, Amon M, Schild G, et al. Inflammation after implantation of hydrophilic acrylic, hydrophobic acrylic, or silicone intraocular lenses in eyes with cataract and uveitis: comparison to a control group. J Cataract Refract Surg. 2002;28:1153-1159.
- Abela-Formanek C, Amon M, Schauersberger J, et al. Uveal and capsular biocompatibility of 2 foldable acrylic intraocular lenses in patients with uveitis or pseudoexfoliation syndrome: comparison to a control group. J Cataract Refract Surg. 2002;28:1160-1172.
- Werner L, Pandey SK, Escobar-Gomez M, Visessook N, Peng Q, Apple DJ. Anterior capsule opacification: A histopathological study comparing different IOL styles. Ophthalmology. 2000;107:463-471.
- Werner L, Pandey SK, Apple DJ, Escobar-Gomez M, McLendon L, Macky T. Anterior capsule opacification: correlation of pathological findings with clinical sequelae. Ophthalmology. 2001;108:1675-1681.
- Kohnen T, Fabian E, Gerl R, et al. Optic edge design as long-term factor for posterior capsular opacification rates. Ophthalmology. 2008;115:1308-1314.
- Werner L, Mamalis N, Izak AM, et al. Posterior capsule opacification in rabbit eyes implanted with 1-piece and 3-piece hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. 2005;31:805-811.
- Nishi O, Nishi K. Preventing posterior capsule opacification by creating a discontinuous sharp bend in the capsule. J Cataract Refract Surg. 1999;25:521-526.
- Boyce JF, Bhermi GS, Spalton DJ, El-Osta AR. Mathematic modeling of the forces between an intraocular lens and the capsule. J Cataract Refract Surg. 2002;28:1853-1859.
- Werner L, Müller M, Tetz M. Evaluating and defining the sharpness of intraocular lenses. Microedge structure of commercially available square-edged hydrophobic lenses. J Cataract Refract Surg. 2008;34:310-317.
- Werner L, Tetz M, Feldmann I, Bücker M. Evaluating and defining the sharpness of intraocular lenses: microedge structure of commercially available square-edged hydrophilic intraocular lenses. J Cataract Refract Surg. 2009;35:556-566.
- Kugelberg M, Wejde G, Jayaram H, Zetterström C. Posterior capsule opacification after implantation of a hydrophilic or a hydrophobic acrylic intraocular lens: one-year follow-up. J Cataract Refract Surg. 2006;32:1627-1631.
- Richter-Mueksch S, Kahraman G, Amon M, Schild-Burggasser G, Schauersberger J, Abela-Formanek C. Uveal and capsular biocompatibility after implantation of sharp-edged hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg. 2007;33:1414-1418.
- Werner L. Glistenings and surface light scattering in intraocular lenses. J Cataract Refract Surg. 2010;36:1398-1420 (Review).
- Ollerton A, Werner L, Fuller SR, Kavoussi SC, McIntyre JS, Mamalis N. Evaluation of a new single-piece 4% water content hydrophobic acrylic intraocular lens in the rabbit model. J Cataract Refract Surg. 2012 (in press).
- Werner L. Causes of intraocular lens opacification or discoloration. J Cataract Refract Surg. 2007;33:713-726 (Review).
- Mamalis N. Complications of foldable intraocular lenses requiring explantation or secondary intervention-2001 survey update. J Cataract Refract Surg. 2002;28:2193-2201.
- Werner L, Apple DJ, Escobar-Gomez M, et al. Postoperative deposition of calcium on the surfaces of a hydrogel intraocular lens. Ophthalmology. 2000;107:2179-2185.
- Werner L, Apple DJ, Kaskaloglu M, Pandey SK. Dense opacification of the optical component of a hydrophilic acrylic intraocular lens: a clinicopathological analysis of 9 explanted lenses. J Cataract Refract Surg. 2001;27:1485-1492.
- Dorey MW, Brownstein S, Hill VE, et al. Proposed pathogenesis for the delayed postoperative opacification of the hydroview hydrogel intraocular lens. Am J Ophthalmol. 2003;135:591-598.
- Neuhann IM, Werner L, Izak AM, et al. Late postoperative opacification of a hydrophilic acrylic (hydrogel) intraocular lens: A clinicopathological analysis of 106 explants. Ophthalmology. 2004;111:2094-2101.
- Haymore J, Zaidman G, Werner L, et al. Misdiagnosis of hydrophilic acrylic intraocular lens optic opacification: report of 8 cases with the MemoryLens. Ophthalmology. 2007;114:1689-1695.
- Werner L, Wilbanks G, Ollerton A, Michelson J. Localized calcification of hydrophilic acrylic intraocular lenses in association with intracameral injection of gas. J Cataract Refract Surg. 2012;38:720-721 (Letter to the Editor).
- Foot L, Werner L, Gills JP, et al. Surface calcification of silicone plate intraocular lenses in patients with asteroid hyalosis. Am J Ophthalmol. 2004;137:979-987.
- Wackernagel W, Ettinger K, Weitgasser U, et al. Opacification of a silicone intraocular lens caused by calcium deposits on the optic. J Cataract Refract Surg. 2004;30:517-520.
- Werner L, Kollarits CR, Mamalis N, Olson RJ. Surface calcification of a three-piece silicone intraocular lens in a patient with asteroid hyalosis: A clinicopathologic case report. Ophthalmology. 2005;112:447-452.
- Stringham J, Werner L, Monson B, Theodosis R, Mamalis N. Calcification of different designs of silicone intraocular lenses in eyes with asteroid hyalosis. Ophthalmology. 2010;117:1486-1492.